Answers
Answer:
the first one
Explanation:
because when a covalent bond happens the product is called a molecule
Covalent compounds are those whose atoms are joined together by covalent bonds. Sharing one or more pairs of valence electrons results in the formation of a covalent bond. The correct option is A.
When two nonmetals interact, covalent or molecular compounds are created. The elements unite to create a compound, which results in an electrically neutral molecule, by sharing electrons.
Strong intramolecular connections are only seen in covalent substances. This is due to the firmly bound nature of the atoms within covalent bonds. Each molecule in a covalent compound is in fact extremely distinct, and there is typically little attraction between them.
Thus the correct option is A.
To know more about covalent compounds, visit;
https://brainly.com/question/11632372
#SPJ3
Related Questions
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
There are four molecules of nitrogen and nine molecules of hydrogen present in the diagram.
When the reaction is complete, how many molecules of NH3 are produced?
What is the limiting reactant?
How many molecules of each reactant are remain after the reaction is complete?
Answers
After the reaction is complete, no nitrogen and no hydrogen molecules remain, and 8.00 x 1014 molecules of NH3 are produced.
In the equation, nitrogen and hydrogen react at a high temperature, in the presence of a catalyst, to produce ammonia, according to the balanced chemical equation:N2(g)+3H2(g)⟶2NH3(g)The coefficients of each molecule suggest that one molecule of nitrogen reacts with three molecules of hydrogen to create two molecules of ammonia.
So, to determine how many molecules of ammonia are produced when four nitrogen and nine hydrogen molecules are present, we must first determine which of the two reactants is the limiting reactant.
To find the limiting reactant, the number of moles of each reactant present in the equation must be determined.
Calculations:
Nitrogen (N2) molecules = 4Hence, the number of moles of N2 = 4/6.02 x 1023 mol-1 = 6.64 x 10-24 mol
Hydrogen (H2) molecules = 9Hence, the number of moles of H2 = 9/6.02 x 1023 mol-1 = 1.50 x 10-23 mol
Now we have to calculate the number of moles of NH3 produced when the number of moles of nitrogen and hydrogen are known, i.e., mole ratio of N2 and H2 is 1:3.
The mole ratio of N2 to NH3 is 1:2; thus, for every 1 mole of N2 consumed, 2 moles of NH3 are produced.
The mole ratio of H2 to NH3 is 3:2; thus, for every 3 moles of H2 consumed, 2 moles of NH3 are produced.
From these mole ratios, it can be observed that the limiting reactant is nitrogen.
Calculation for NH3 production:
Nitrogen (N2) moles = 6.64 x 10-24 moles
The mole ratio of N2 to NH3 is 1:2; therefore, moles of NH3 produced is 2 × 6.64 × 10−24 = 1.33 × 10−23 moles.
Now, to determine how many molecules of NH3 are produced, we need to convert moles to molecules.
1 mole = 6.02 x 1023 molecules
Thus, 1.33 x 10-23 moles of NH3 = 8.00 x 1014 molecules of NH3 produced.
To find the amount of each reactant remaining after the reaction is complete, we must first determine how many moles of nitrogen are consumed, then how many moles of hydrogen are consumed, and then subtract these from the initial number of moles of each reactant.
The moles of nitrogen consumed = 4 moles × 1 mole/1 mole N2 × 2 mole NH3/1 mole N2 = 8 moles NH3
The moles of hydrogen consumed = 9 moles × 2 mole NH3/3 mole H2 × 2 mole NH3/1 mole N2 = 4 moles NH3
Thus, the moles of nitrogen remaining = 6.64 × 10−24 mol – 8 × 2/3 × 6.02 × 10^23 mol-1 = 5.06 × 10−24 mol
The moles of hydrogen remaining = 1.50 × 10−23 mol – 4 × 2/3 × 6.02 × 10^23 mol-1 = 8.77 × 10−24 mol
Finally, the number of molecules of each reactant remaining can be calculated as follows:
Number of N2 molecules remaining = 5.06 × 10−24 mol × 6.02 × 10^23 molecules/mol = 3.05 × 10−1 molecules ≈ 0 molecules
Number of H2 molecules remaining = 8.77 × 10−24 mol × 6.02 × 10^23 molecules/mol = 5.28 × 10−1 molecules ≈ 0 molecules.
For more such questions on molecules
https://brainly.com/question/24191825
#SPJ8
The measure of the length of events and the duration of intervals between events
Answers
The measure of the length of events and the duration of intervals between events is time.
What is time?The duration of events or the gaps between them can be measured, compared, or even ordered using time. The lengthy period of time that the Earth's geologic history takes up is known as geologic time. Starting at the beginning of the Archean Eon formal geologic time runs until the present. Geology is defined as the "Science of the Earth."
Geology is the fundamental Earth science that examines how the earth created, its structure and composition, and the various forces acting on it. It is sometimes known as geoscience or earth science.
Learn more about time at;
https://brainly.com/question/479532
#SPJ1
Hydrogen reacts with oxygen according to the balanced equation
2H₂ (g) + O2(g) → 2H₂O(g). If X is the number of molecules of H₂ which react,
then the number of O2 molecules reacting is
Answers
Answer:
x/2
Explanation:
X = 2 molecules of H2
For 2 molecules of H2, there's only 1 molecule of O2. Meaning, there's twice the amount of H2, so O2 = x/2 molecules.
I hope I'm understanding this question right.
A rock has an area of 2370 in.² what is the area in square centimeters
Answers
Answer:
VIDEO ANSWER: This question, we're starting with 2370 square inches, and we want to convert this to square centimeters.
Explanation:
.
Based on the thermodynamic properties provided for water, determine the energy change when the temperature of 0.950 kg of water decreased from 103 °C to 60.5 °C.
Property Value Units
Melting point 0 °C
Boiling point 100.0 °C
ΔHfus 6.01 kJ/mol
ΔHvap 40.67 kJ/mol
cp (s) 37.1 J/mol · °C
cp (l) 75.3 J/mol · °C
cp (g) 33.6 J/mol · °C
Answers
The energy change when the temperature of 0.950 kg of water decreased from 103 °C to 60.5 °C is 2308.87 kJ.
What is the heat energy change when the temperature of 0.950 kg of water decreased from 103 °C to 60.5 °C?The heat energy change when the temperature of 0.950 kg of water decreased from 103 °C to 60.5 °C is determined from the formulas below:
Heat change 1 = Heat capacity as gas * moles * temperature change
Heat change 2 = Heat of vaporization, ΔHvap * moles
Heat change 3 = Heat capacity as liquid, Cp (l) * moles * temperature change
moles of water = 950 kg * 1000 g/kg * 1 mol/ 18g = 52.78 mole
Heat change 1 = 33.6 * 52.78 * (103 - 100) = 5320.224 J
Heat change 2 = 40.67 * 1000 * 52.78 = 2146562.6 J
Heat change 3 = 75.3 * 52.78 * (100 - 60.5) = 156986.193 J
Total heat change = 5320.224 J + 2146562.6 J + 156986.193 J
Total heat change = 2308869.017 J = 2308.87 kJ
Learn more about heat change at: https://brainly.com/question/14047927
#SPJ1
What is the correct formula that would result from the combination of the two ionic species? Cu2+ and SO42-
Answers
CuSO4.
brainliest???
what do large scale convection currents create science
Answers
Large-scale convection currents create divergent plate boundaries in science.
What is large scale convection currents?This refers to vertical motion organized on a larger scale than atmospheric free convection, it is associated with cumulus clouds. Examples of large-scale convection currents patterns of vertical motion is hurricanes or migratory cyclones.
Large convection currents in the aesthenosphere send heat to the surface, where plumes of less dense magma break apart the plates at the spreading centers, giving rise to divergent plate boundaries.
Learn more about Large-scale convection currents on
https://brainly.com/question/8560104
#SPJ1
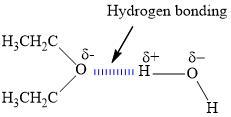
2. How many hydrogen bonds can form between a single ether molecule and water molecules? Draw the structures to explain.
Answers
Ether can only form one hydrogen bond per molecule.
What is hydrogen bonding?We know that the hydrogen bond is the kind of bond that occurs when the dipole of water interacts with the dipole that is on another molecule. We can see this in a lot of hydrides of electronegative elements.
We can see that ether has only one electronegative atom and that is oxygen from the image that is shown in the answer. This oxygen atom can interact with the positive end of the dipole in only one water molecule at a time.
Learn more about hydrogen bonding:https://brainly.com/question/15099999
#SPJ1
How many moles of aluminum ions al3+ are present in 0.42 mol of al2so43
Answers
There are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
To determine the number of moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3, we need to consider the stoichiometry of the compound.
The formula of aluminum sulfate (Al2(SO4)3) indicates that for every 1 mole of the compound, there are 2 moles of aluminum ions (Al3+). This means that the mole ratio of Al3+ to Al2(SO4)3 is 2:1.
Given that we have 0.42 mol of Al2(SO4)3, we can calculate the moles of Al3+ as follows:
Moles of Al3+ = 0.42 mol Al2(SO4)3 x (2 mol Al3+ / 1 mol Al2(SO4)3)
Moles of Al3+ = 0.42 mol Al2(SO4)3 x 2
Moles of Al3+ = 0.84 mol Al3+
Therefore, there are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
It's important to note that the stoichiometry of the compound determines the mole ratio between the different species involved in the chemical formula. In this case, the 2:1 ratio of Al3+ to Al2(SO4)3 allows us to determine the number of moles of Al3+ based on the given amount of Al2(SO4)3.
For more such question on aluminum visit:
https://brainly.com/question/30451292
#SPJ8
If the distance the pepper flakes moved were a measure of the strength of the intermolecular force, would you say the effect was stronger or weaker in hand sanitizer compared to soap
Answers
Answer:
In the given case, the pepper flakes are considered virus particles. One of the applications of the principle of "like dissolves like" is the behavior of hand sanitizers and soaps. The polar molecules like alcohols comprise -OH as the functional groups, which are fascinated towards the water, thus, producing robust intermolecular associations. In comparison, the nonpolar molecules like fats and oils, proteins comprise hydrocarbon groups, which are hydrophobic to water.
The active constituents of both hand sanitizers and soaps show the features of both nonpolar and polar regions in their molecular compositions. As a consequence, part of the molecule is fascinated by water, while some parts are attracted towards organic molecules like fats and proteins.
The virus particles are enveloped with different kinds of proteins and fats, so when soaps and hand sanitizers come in contact with the virus, the nonpolar regions of sanitizers and soaps get fascinated towards the coating, and efficiently pulls apart the virus composition.
In the given case, one can model the principle by assuming pepper flakes as virus particles, which are coated with fats and proteins, and thus are insoluble in water. After this, one can simultaneously see the effect of soaps and sanitizers by using them one by one.
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
Help please asap will mark brainliest!

Answers
Answer:
28.466256
Explanation:
A non-zero electron dipole moment for this particle would violate CPT symmetry. The results of an earlier experiment by Irene and Frederic Joliot-Curie involving polonium, beryllium and paraffin wax was explained as the emission of this particle. Because its' radiative capture cross-section has no regions of resonance, Boron-10 is used to control the(*) radiation of these particles in thermal reactors. They consist of one up and two down quarks. Isotopes have the same number of protons, but different numbers of these particles. For 10 points, name these particles discovered by James Chadwick, which have no electric charge.
Answers
Neutrons are the particles that James Chadwick first identified as having no electric charge. Isotopes have variable numbers of neutrons despite having the same amount of protons.
How do protons work?The positively charged particles called protons are found in the atom's nucleus. The strong force, which is stronger at short distances, pulls the protons together while the electromagnetic force pushes them apart.
How do isotopes work?Isotopes are variations of chemical elements that have a varied number of neutrons but the same number of protons and electrons. To put it another way, isotopes are variations of elements that have different nucleons.
To learn more about atoms visit:
brainly.com/question/13654549
#SPJ4
Which statements describe the nature of science? (Select 5)
1. Scientists engage in peer reviews to avoid bias.
2. Science is a blend of logic and innovation.
3. Scientific ideas are not durable and cannot adjust to change as new data is collected.
4. Science is not observational .
5. Science is a complex social endeavor.
6. Natural world is understandable.
7. Scientists try to remain objective.
Answers
Answer:
2
Explanation:
Scientists engage in peer reviews to avoid bias, Science is a blend of logic and innovation and Scientific ideas are not durable and cannot adjust to change as new data is collected. The correct options are 1,2, and 7.
Peer reviews are used by scientists to ensure objectivity and reduce prejudice in their study. To create original ideas and hypotheses, scientists need both logical reasoning and creative thinking.
As scientists frequently interact, exchange ideas, and build on one another's work, it is a complex social endeavour. Understanding and making sense of the natural world is the central tenet of science.
Finally, scientists separate their personal beliefs from empirical evidence in order to stay as objective as possible in their research.
Thus, Scientists engage in peer reviews to avoid bias, Science is a blend of logic and innovation and Scientific ideas are not durable and cannot adjust to change as new data is collected. The correct options are 1,2, and 7.
Learn more about Nature, refer to the link:
https://brainly.com/question/30406208
#SPJ7
3. A
always a compound.
is always a molecule, but a is not always a compound.
Answers
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
When a small amount of 12 M HNO3(aq) is added to a buffer solution made by mixing CH3NH2(aq) and CH3NH3Cl(aq) , the pH of the buffer solution changes from 10.64 to 10.62. Which of the following equations represents the reaction that accounts for the fact that the pH does not change significantly when the HNO3(aq) is added?
a. CH3NH2(aq) + H+(aq) â CH3NH3+(aq)
b. CH3NH3+(aq) + H+(aq) â CH3NH42+(aq)
c. NO3- (aq) + H+(aq) â HNO3(aq)
d. OH- (aq) + H+(aq) â H2O(l)
Answers
Answer:
a. CH3NH2(aq) + H⁺ → CH3NH3⁺
Explanation:
The mixture of a weak base as CH3NH2 with its conjugate acid CH3NH3Cl produce a buffer. As the weak acid is in equilibrium with water, the mixture of the weak base and its conjugate base produce that the acid or base released react avoiding the change in pH.
For example, when a strong acid as HNO3 reacts, the weak base will react producing the conjugate base, that is:
CH3NH2(aq) + H⁺ → CH3NH3⁺
Right answer is:
a. CH3NH2(aq) + H⁺ → CH3NH3⁺The single strand of nucleic acid shown is representative of
A). RNA
B). DNA
C). both RNA and DNA
D). protein

Answers
1. Answer the following questions regarding the peptide LCYRAIDCG a) What is the sequence of amino acids written as the THREE letter code? b) Draw the structure of the peptide LCYRAIDCG as it would appear at pH 5 under oxidizing conditions. Be sure to include any disulfide bonds that would form. c) Label the N-terminus of the peptide in b) with a tick (√) d) Label the C-terminus of the peptide you drew in b) with a star (*)
Answers
b) Drawing the structure of the peptide LCYRAIDCG at pH 5 under oxidizing conditions with disulfide bonds:
H3N+ - Leu - Cys(S-S)- Tyr - Arg - Ala - Ile - Asp - Cys(S-S)- Gly - COO-
c) The N-terminus of the peptide in b) would be labeled with a tick (√) at the amino group (H3N+).
d) The C-terminus of the peptide in b) would be labeled with a star (*) at the carboxyl group (COO-).
When 1.04g of cyclopropane was burnt in excess oxygen in a bomb calorimeter, the temperature rose by 3.69K. The total heat capacity of the calorimeter and it's contents was 14.01kJ/K. Determine the enthalpy of combustion of cyclopropane.
Answers
Answer:
\(\Delta _{comb}H=-2,093\frac{kJ}{mol}\)
Explanation:
Hello!
In this case, since these calorimetry problems are characterized by the fact that the calorimeter absorbs the heat released by the combustion of the substance, we can write:
\(Q_{rxn}+Q_{cal}=0\)
Thus, given the temperature change and the total heat capacity, we obtain the following total heat of reaction:
\(Q_{rxn}=-14.01kJ/K*3.69K\\\\Q_{rxn}=-51.70kJ\)
Now, by dividing by the moles in 1.04 g of cyclopropane (42.09 g/mol) we obtain the enthalpy of combustion of this fuel:
\(n=\frac{1.04g}{42.09g/mol}=0.0247mol\\\\\Delta _{comb}H=\frac{Q_{rxn}}{n}\\\\ \Delta _{comb}H=-2,093\frac{kJ}{mol}\)
Best regards!
The enthalpy of combustion of cyclopropane is \(\Delta _c_o_m_b H= - 2.093 kJ/mol\)
What is the enthalpy of combustion?
Enthalpy of combustion is the amount of heat produced when one mole of substance completely burns.
By the formula of calorimetry
\(Q_r_x_n + Q_c_a_l = 0\)
The temperature change and total heat capacity is
\(Q_r_x_n = -14.01 KJ/K \times 3.69 K\\\\Q_r_x_n = -51.70 KJ\)
Now, we get the enthalpy of combustion by dividing by the moles of 1.04 g of cyclopropane.
The number of moles is 42.09 g/mol.
\(n = \dfrac{1.4}{42.09} = 0.0247\\\\\Delta_c_o_m_bH =\dfrac{Q_r_x_n}{n} \\\\\Delta_c_o_m_bH = -2.093\; kJ/mol\)
Thus, the enthalpy of combustion is -2.093 kJ/mol
Learn more about enthalpy of combustion
https://brainly.com/question/8261033
Solve the equation by first using the Quadratic Formula and then by factoring.
x2 – 14x + 48 = 0
Answers
X=8,6
3. A student obtained the following data using the procedure used in this experiment.
BEFORE IGNITION
mass of crucible + crucible cover = 34.12 g
mass of crucible + cover + unknown sample = 36.65 g
AFTER IGNITION
mass of crucible + cover + unknown (1st weighing) = 35.67 g
mass of crucible + cover + unknown (2nd weighing) = 35.23 g
mass of crucible + cover + unknown (3rd weighing) = 35.20 g
Calculate the % water in the unknown sample:_______?
Answers
The percent of water in the unknown sample is calculated as 57.3%.
What is ignition?Process of providing energy that is required to initiate a combustion process is called ignition.
mass of crucible + cover + unknown sample = 36.65 g
mass of crucible + cover = 34.12 g
mass of unknown sample = (36.65 g - 34.12 g) = 2.53 g
mass of water lost during ignition:
mass of unknown sample (before ignition) = 2.53 g
mass of unknown sample (after 1st weighing) = 35.67 g - 34.12 g = 1.55 g
mass of unknown sample (after 2nd weighing) = 35.23 g - 34.12 g = 1.11 g
mass of unknown sample (after 3rd weighing) = 35.20 g - 34.12 g = 1.08 g
total mass of water lost during ignition = (2.53 g - 1.08 g) = 1.45 g
As % water in unknown sample = (mass of water lost / mass of unknown sample) x 100%
= (1.45 g / 2.53 g) x 100%
% water in unknown sample = 57.3%
Therefore, percent of water in the unknown sample is 57.3%.
To know more about ignition, refer
https://brainly.com/question/28270114
#SPJ1
how are digital road maps different from paper and rod map?
a. they allow users to plan routes before a trip
b. they can be used anywhere in the world
c. they can be updated almost immediately
d. they show major and minor roads in a region
please helppppp need it asap
Answers
Digital road maps are different from paper and road maps in that they allow users to plan routes before a trip, they can be used anywhere in the world, and they can be updated almost immediately. They also show both major and minor roads in a region.
Digital road maps are a type of digital map that is based on GIS data. This data is collected from various sources and is used to create a detailed representation of the real world. The digital road map allows the user to zoom in and out and to see different levels of detail, depending on their needs. They also allow the user to search for specific locations, find directions, and plan routes before they begin their trip.
On the other hand, paper maps are usually printed on paper and can be difficult to read in low light conditions. They are also limited in the amount of detail that they can show, and they may not always be up-to-date.
Road maps, on the other hand, are a type of map that shows roads and highways in a region. They may include some additional features such as rest areas, gas stations, and other points of interest. Road maps are typically printed on paper and are often used for navigation while driving. They are not as detailed as digital road maps, and they can quickly become outdated as new roads are built.
For more such questions on Digital road maps
https://brainly.com/question/12456226
#SPJ8
How many grams of Al2O3
can form from 37.7 g
of Al
?
4Al(s)+3O2(g)⟶2Al2O3(s)
Answers
71.26 g of Al2O3 can form from 37.7 g of Al.
How to determine how many grams of Al2O3 can form from 37.7 g of AlThe balanced chemical equation is:
4Al(s) + 3O2(g) ⟶ 2Al2O3(s)
From the equation, we can see that 4 moles of Al react with 3 moles of O2 to form 2 moles of Al2O3. This means that the mole ratio of Al to Al2O3 is 4:2 or 2:1.
To determine how many grams of Al2O3 can form from 37.7 g of Al, we need to use the mole ratio and the molar mass of Al2O3.
Calculate the number of moles of Al:
molar mass of Al = 26.98 g/mol
moles of Al = mass/molar mass = 37.7 g / 26.98 g/mol = 1.397 mol
Use the mole ratio to determine the number of moles of Al2O3 that can form:
moles of Al2O3 = 1.397 mol Al × (2 mol Al2O3 / 4 mol Al) = 0.6985 mol Al2O3
Calculate the mass of Al2O3 using its molar mass:
molar mass of Al2O3 = 101.96 g/mol
mass of Al2O3 = moles of Al2O3 × molar mass = 0.6985 mol × 101.96 g/mol = 71.26 g
Therefore, 71.26 g of Al2O3 can form from 37.7 g of Al.
Learn more about mole ratio here : brainly.com/question/30851942
#SPJ1
Can someone please help me with this question. I got half of the question and I am stuck on the rest.

Answers
The mean of the data set is approximately 4.0626, and the 90% confidence interval is [4.060925, 4.064275].
What is the mean and 90% confidence interval of the given data?The sample mean (x) is calculated as follows:
x = (4.0620 + 4.0550 + 4.0650 + 4.0740 + 4.0550 + 4.0660) / 6
x ≈ 4.0626 (rounded to four decimal places)
The 90% confidence interval is calculated as follows;
Standard deviation (s):
(4.0620 - 4.0626)² = 0.00000036
(4.0550 - 4.0626)² = 0.00000576
(4.0650 - 4.0626)² = 0.00000006
(4.0740 - 4.0626)² = 0.00001328
(4.0550 - 4.0626)² = 0.00000576
(4.0660 - 4.0626)² = 0.00000012
average of the squared differences:
(0.00000036 + 0.00000576 + 0.00000006 + 0.00001328 + 0.00000576 + 0.00000012) / 6 ≈ 0.00000624
s = √(0.00000624)
s ≈ 0.002496
the standard error of the mean (SEM):
SEM = 0.002496 / √6
SEM ≈ 0.001018
For a 90% confidence interval, the z value is approximately 1.645.
ME = 1.645 * 0.001018 ≈ 0.001675
CI = x ± ME
CI = 4.0626 ± 0.001675
CI ≈ [4.060925, 4.064275]
Learn more about mean and confidence intervals at: https://brainly.com/question/20309162
#SPJ1
The volume of a gas is 590 mL at 384 kPa pressure. What will the volume be when the pressure is changed to 546 kPa, assuming the temperature
remains constant?
Answers
Answer:
The answer is 414.95 mLExplanation:
To find the volume when the pressure is changed to 546 kPa , we use the formula for Boyle's law which is
\(P_1V_1 = P_2V_2\)
where
P1 is the initial pressure
P2 is the final pressure
V1 is the initial volume
V2 is the final volume
Since we are finding the final volume
\(V_2 = \frac{P_1V_1}{P_2} \\\)
We have
\(V_2 = \frac{384000 \times 590}{546000} = \frac{226560000}{546000} \\ = 414.945054...\)
We have the final answer as
414.95 mLHope this helps you
Will ag2so4 precipitate when 100ml of .050M agno3 is mixed with 10ml of 5x10-2m na2so4 solution
Answers
No, Ag₂SO₄ will not precipitate when 100ml of .050M AgNO3 is mixed with 10ml of 5x10-2 m Na₂SO₄ solution because the precipitate is made only in an aqueous solution.
What is precipitation?Precipitation is the solid extract that is collect in a place. Precipitate is the concentration of the substance in a solution in a specific place.
Thus, No, Ag₂SO₄ will not precipitate when 100ml of .050M AgNO3 is mixed with 10ml of 5x10-2 m Na₂SO₄ solution because the precipitate is made only in an aqueous solution.
Learn more about precipitation
https://brainly.com/question/18109776
#SPJ1
Did all the silver ions get consumed in the reaction? The molar mass of silver is 107.87 g/mol. Justify your answer.
Answers
This problem is asking for the consumption of silver ions when silver nitrate is reacted with copper. In such a case, since no masses are given, we can use the following from similar problems:
Mass of empty beaker: 110.000 g
Mass of beaker with silver nitrate (after all additions) and copper: 331.634 g.
Mass of beaker with silver: 113.395 g.
This means we can write the following chemical equation:
\(Cu+2AgNO_3\rightarrow Cu(NO_3)_2+2Ag\)
And thus calculate the mass of silver nitrate that will produce the following mass of silver:
\(m_{Ag}^{produced}=113.395g-110.000g=3.395g\)
Next, we use the 2:2 mole ratio of silver to silver nitrate (silver ions source):
\(3.395gAg*\frac{1molAg}{107.87gAg}*\frac{2molAgNO_3}{2molAg} *\frac{169.87gAgNO_3}{1molAgNO_3} = 5.35gAgNO_3\)
The step will be defined for the given mass of available silver nitrate which will be compared to 5.35 g (consumed mass) to see if they are the same (all consumed) or different (partial consumption).
Learn more:
https://brainly.com/question/22031122https://brainly.com/question/2607181https://brainly.com/question/16965188Check all that apply...helppppp

Answers
Answer:
dfgh
Explanation:
Students were shown models of two atoms and asked to make a list of similarities and differences between the models.
Which of the statements about the atomic models shown is correct?
A) Both models represent atoms with the same atomic number.
B) Both models represent atoms with the same atomic mass.
C) The model on the left is an ion and the model on the right is an isotope.
D) The model on the left is an isotope and the model on the right is an ion.

Answers
Answer:
A: Both models represent atoms with the same atomic number.
Explanation:
The atomic number equals the number of protons.
The correct statement is that both models represent atoms with the same atomic number.
What are isotopes?Isotopes are those molecules which are having the same atomic numbers but different atomic masses.
In the given model both they have the same number of electrons and protons it means they are not ions, but are having different atomic masses as number of neutrons are different.
Hence both models represent atoms with the same atomic number.
To know more about isotopes, visit the below link:
https://brainly.com/question/14220416
#SPJ2