Answers
The volume of the liquid in this diagram shown above would be equal to 36.5 mL.
What is a graduated cylinder?A graduated cylinder is also known as measuring cylinder and it can be defined as a narrow, cylindrical piece of laboratory equipment with marked lines, which are used to measure the volume of a liquid.
In order to take a reading for the measurement of the volume of a liquid such as water, you should ensure that your eye level is even with the center of the meniscus.
In this scenario, the volume of the liquid in this diagram would be 36.5 mL because each of the small lines on the graduated cylinder measures 0.5 mL.
Read more on graduated cylinder here: https://brainly.com/question/24869562
#SPJ1
Related Questions
2. Which state of matter is characterized by particles that are close to each other but are not arranged in a definite pattern?
A)liquid
B)plasma
C)solid
D)gas
Answers
Answer:
Solid
Explanation:
Cus its solid, take a brick for example. It's hard and has no space unlike liquid or gas.
CH3 H H CH3
| | | |
CH3--- C -------C------ C -------C--CH3
| | | |
CH2 CH2 CH2 CH2
| | | |
CH3 CH3 CH3 CH3
what is the name of this compund
Answers
The systematic name of the compound is 2,2,3,3-tetramethylbutane.
What is the name of this compound ?The parent chain in this compound consists of four carbon atoms, indicated by the central chain of Cs in the diagram you provided. The prefix "but" in the name indicates that there are four carbon atoms in the parent chain.
The four methyl groups are attached to different carbon atoms in the parent chain. We can indicate the position of each methyl group by numbering the carbons in the parent chain. In this case, we can number the carbons starting from the left side of the parent chain, so that the first methyl group is attached to the second carbon, the second methyl group is attached to the third carbon, the third methyl group is attached to the second-to-last carbon, and the fourth methyl group is attached to the last carbon. This gives us the substituent groups 2,2,3,3-tetramethyl-.
Finally, we combine the parent chain name and the substituent groups to get the full name: 2,2,3,3-tetramethylbutane.
Learn more about organic compounds here: https://brainly.com/question/26854014
#SPJ1
Some confusion is introduced by the fact that physicists might focus on an individual molecule whereas a chemist might focus on a mole of them (1 mole = 6.023 x 10^{23} molecules or atoms).
If a particular molecule had a bond dissociation energy of 1 eV, how much energy would be needed (in kJ) to break all the bonds in one mole of molecules (not yet considering any interactions with the environment)?
Answers
To break all the bonds in one mole of molecules with a bond dissociation energy of 1 eV, we would require around 5.80 x \(10^{26}\) kJ of energy.
The bond dissociation energy of 1 eV is roughly 96.485 kJ/mol. To break all the bonds in one mole of molecules (6.023 x \(10^{23}\) molecules), we would need to multiply the bond dissociation energy by Avogadro's number:
96.485 kJ/mol x 6.023 x \(10^{23}\) molecules/mol = 5.80 x \(10^{26}\) kJ/mol
Bond dissociation energy is the amount of energy necessary to break a chemical connection between two atoms. It is also known as bond energy or bond enthalpy. This energy is measured in kilojoules per mole (kJ/mol) and is released when the bond forms. The energies of bond dissociation can vary greatly depending on the type of bond being broken.
For more such questions on enthalpy, click on:
https://brainly.com/question/14047927
#SPJ4
Which is an unreliable source?
A scientist who studies dinosaurs presents her research on velociraptors at a science convention.
An advertisement for acne medication claims it can clear up acne in less than one week.
An article on the Centers for Disease Control and Prevention (CDC) website discusses the salmonella bacteria.
A chemistry teacher discusses possible projects for the upcoming science fair.
Answers
The source that is unreliable is an advertisement for acne medication claims it can clear up acne in less than one week.
What are citable sources?Citable sources are those sources that are gotten from places that are proven to be of good standards and not from mere claims such as:
Science journalsprofessional organizationsgovernment agenciesTherefore, the source that is unreliable is an advertisement for acne medication claims it can clear up acne in less than one week.
Learn more about citations here:
https://brainly.com/question/8130130
#SPJ1
Water (2470 g ) is heated until it just begins to boil. If the water absorbs 5.47x105 J of heat in the process,
what was the initial temperature of the water?
Express your answer with the appropriate units.
Answers
Answer:
47.01 °C
Explanation:
Q = mcΔt
Mass of water = 2470g
c or specific heat capacity of water = 4.18 J °C g
Q = 5.47 x 10⁵ J, which is expanded 547,000 J
No idea what Δt is, but we do know the water began to boil, and we know that water's boiling point is 100°C, so the final temperature had to be 100°C.
547,000 J = (2470) x (4.18) x (Δt)
Rearrange equation for Δt
(547,000) / (2470 x 4.18) = Δt
Δt = 52.98°C
But we're not done. We're trying to find the INITIAL TEMPERATURE of the water, not the TEMPERATURE CHANGE. We already have the final temperature, which is 100°C, and now we have how much the initial temperature rose by to get to 100°C.
All that we have to do now is 100 - 52.98 = 47.02°C
The initial temperature of the water is 47.02°C
100 Points! PLZ HELP
Which statement best describes the model below?

Answers
the image shows the moles of gas increasing as well as volume increasing
Answer:
Option D
Explanation:
Because we know the relationship between no of moles and volume
Volume is directly proportional to no of molesSo if moles is increased volume would be increased too .Option D
25 g of iron is added to a graduated cylinder containing 45 mL of water. The water level rises to the 50 mL mark. From this information, calculate the density of the iron.
Answers
Answer:
imp not sure but i think the density is 5
Explanation:
im probably wrong but i think this because the water rose to the 50 ml mark when before it was 45 then again i am only in 8th grade but it makes sense to me
Lets make this one quick.

Answers
You are offered a case of brandy supposedly bottled in the time of Napoleon for a really great price. Before buying it, you insist on testing a sample of the brandy and find that it has a tritium content of that of newly produced brandy. How long ago was the brandy bottled? Is it likely to be authentic Napoleon-era brandy?
Answers
The most important thing to remember about tritium sights is that they gradually lose their effectiveness over time. Tritium has a half-life of about 12.5 years, so tritium sights will only be half as bright in a little more than a decade.
What is the lifetime of tritium?Tritium is radioactive and has a half-life of approximately 12.5 years, which means that half of the radioactive atoms will naturally decay during that time. Although tritium can exist as a gas under controlled conditions, it is more commonly found as a liquid, because tritium, like hydrogen, reacts with oxygen to form water.The most important thing to remember about tritium sights is that they gradually lose their effectiveness over time. Tritium has a half-life of about 12.5 years, so tritium sights will only be half as bright in a little more than a decade.To learn more about : Tritium
Ref : https://brainly.com/question/10015637
#SPJ9
1) In the nuclear equation below, what does the letter X represent? Show your work.

Answers
Tells how many of each atom there are in a formula
Answers
Answer:
\(\large \boxed{\mathrm{subscripts}}\)
Explanation:
Subscripts are numbers written after elements, subscripts indicate how many of each atom there are in a compound.
The atoms of each element in an compund is written in the subscript. The number of atoms present in any compound whether made up of only same element or different element is termed as Atomicity.
☃️ For Example:Given compound = \(\boxed{\sf{H_2O}}\)
Here, there are two atoms of hydrogen and one atom of oxygen, So ratio of the atoms of elements in Water molecule = 2 : 1
━━━━━━━━━━━━━━━━━━━━
Please help!!
How many O2 molecules occupy a 1.0 L flask at 65°C and
103.7 kPa?
Select one:
O a. 28 molecules
O b. 2.2 x 1022 molecules
O c.
1.1 x 1023 molecules
O d.
1.6 x 1025 molecules
e. 1.7 x 1025 molecules

Answers
Answer:
B, 2.22×10^22 molecules
Explanation:
Given PV=nRT, n=PV/RT
n=103.7×1.0/8.314×(65+273.15)
= 0.0368858... moles
Given n= number of particles/avogadros number
number of particles=n×avogadros number
number of particles = 0.03688...×6.02x10^23
= 2.22×10^22 molecules
I want to know how to solve this question by step by step.
Question: What is the pH value of 500ml of an aqueous solution of 0.005 mol HCl ?
Answers
The pH value of 500 mL of an aqueous solution of 0.005 mole HCl is 2
How do I determine the pH of the solution?We'll begin our calculations by obtaining the molarity of the solution. Details below:
Volume of solution = 500 mL = 500 / 1000 = 0.50 LMole of HCl = 0.005 moleMolarity = ?Molarity = mole / volume
Molarity = 0.005 / 0.50
Molarity = 0.01 M
Next, we shall obtain the concentration of the hydrogen ion, H⁺ in the solution. Details below:
HCl(aq) <=> H⁺(aq) + Cl¯(aq)
From the balanced equation above,
1 mole of HCl contains 1 mole of H⁺
Therefore,
0.01 M HCl will also contain 0.01 M H⁺
Thus, the concentration of the hydrogen ion, H⁺ in the solution is 0.01 M
Finally, we shall determine the pH of the solution. Details below:
Concentration of hydrogen ion [H⁺] = 0.01 MpH of solution =?pH = -Log H⁺
pH = -Log 0.01
pH = 2
Thus, we can conclude that the pH of the solution is 2
Learn more about pH:
https://brainly.com/question/22983829
#SPJ1
Which of these shows the correct hierarchical sequence?
organs cells ▸ tissues ► organ systems
cells ► tissues ► organs organ systems
organ systems tissues › cells organs
tissues cells organs ► organ systems
Answers
Answer:
cells -> tissues -> organs -> organ system
Answer:
Cells > tissues > Organs > Organs Systems
Explanation:
tissues are made of a group of cells
a specific organ is made up of a geoup of specific type of tissues
and a group of organs make up the organs system
1. How many atoms are in 0.25 moles of carbon?
2. How many atoms are in 12.3 grams of sodium?
3. How many grams are there in 0.52 moles of boron?
4. How many grams are there in 2.0 moles of HCl?
5. How many moles are in 3.4 grams of HBr?
6. How many grams are there in 4.5x10^10 atoms of NaCl?
7. How many atoms are there in 45.1 grams of MgO?
Answers
Answer 1 mole = 6.02 × 1023 atoms
0.25 moles × (6.02 × 1023) = 1.5 × 1023 atoms Carbon
for the first one
Calculate the formula weight or molecular for the following:
a. LiCI
b. SO2 (The 2 is in subscript)
Answers
Answer:
42.39, 64.06
Explanation:
Formula Weight can be calculated by adding the atomic mass of the elements in the formula.
LiCl
AM for Li is 6.94 amu, AM for Cl is 35.45 amu
\((6.94)+(35.45)=42.39\)
SO₂
AM for S is 32.06 amu, AM for O is 16 amu
\((32.06)+2(16.00)=64.06\)
PLX HELP ASAP
How many grams of ethanol, C2H6O, are needed to prepare a 0.100 molal solution using 2.20 kg water?
A) 18.3 g
B) 10.1 g
C) 9.5 g
D) 20.6 g
Answers
Answer:
B) 10.1g
Explanation:
Answer:
10.1 g
Explanation:
N2 + H2 -> NH3
How many moles of H2 gas are used to make 36.5 grams of NH3?
Answers
Explanation:
First, you need to balance the equation:
N2 + 3 H2 ====> 2 NH3
so three moles of H2 result in 2 moles of NH3
ratio of 3:2
How many moles of NH3 is 36.5 gm ??
Using periodic table NH3 mole weight = 14.007 + 3*1.008 =17.031 g/mole
36.5 g / 17.031 g/mole = 2.14 moles of NH3
Using the ratios above 3/2 = x / 2.14 shows x = 3.21 moles of H2 needed
I am a teacher in a college in a large city in China. One day, some students asked me to join
them for dinner. They said they wanted to take me to a very special restaurant that had
just opened. This new place served American food. The students were proud that their city
had a restaurant that served fine American food. I was quite surprised when we got to the
restaurant. My students had taken me to a very well-known fast food restaurant!
1.
In the paragraph above, the author's purpose is to
А. inform.
B
entertain
share personal experiences
C
D
persuade
Answers
Answer:
c or b
Explanation:
the story is entertaining and it shares the teachers personal experiences with the teachers students
Suppose a student repeats Experiment 1 using strontium instead of magnesium. The student adds 4.93 g of strontium to a crucible, heats the crucible and its contents for several minutes over a Bunsen burner, and records the final mass of the crucible and its contents.
Write the balanced chemical equation for this reaction. Include physical states.
balanced equation:
What mass of product is expected to form in this reaction? Assume all of the strontium reacts.
mass of product:
Answers
The balanced chemical equation for the reaction between strontium and oxygen can be written as follows: 2 Sr (s) + \(O_2\)(g) → 2 SrO (s).
In this equation, solid strontium (Sr) reacts with gaseous oxygen (\(O_2\)) to produce solid strontium oxide (SrO).
To determine the mass of product expected to form in this reaction, we need to consider the molar ratio between strontium and strontium oxide. From the balanced equation, we can see that 2 moles of strontium react to produce 2 moles of strontium oxide.
The molar mass of strontium (Sr) is 87.62 g/mol, and the molar mass of strontium oxide (SrO) is 119.62 g/mol. Since the molar ratio is 1:1 between strontium and strontium oxide, the mass of strontium oxide formed will be equal to the mass of strontium used.
In this case, the student added 4.93 g of strontium to the crucible. Therefore, the expected mass of strontium oxide formed will also be 4.93 g.
It's important to note that this calculation assumes that the reaction proceeds to completion, meaning that all of the strontium reacts with oxygen. In actual laboratory conditions, the yield of the reaction may be less than 100% due to factors such as incomplete reaction, side reactions, or product loss.
For more such questsion on balanced chemical equation visit:
https://brainly.com/question/11904811
#SPJ8
You have three gases in a mixture where P1= 100 kPa, P2 = 50 kPa, and P3 = 75
kPa. What is the total pressure of the gas mixture?
A. 225 kPa
B. 25 kPa
C. 75 kPa
D. None of the above
Answers
Answer:
Ptotal=P1+P2+… +Pn. + P nExplanation:
its c
what is the mass of an iridium sample which absorbs 895 j of energy when its temperature increases by 12.3 C
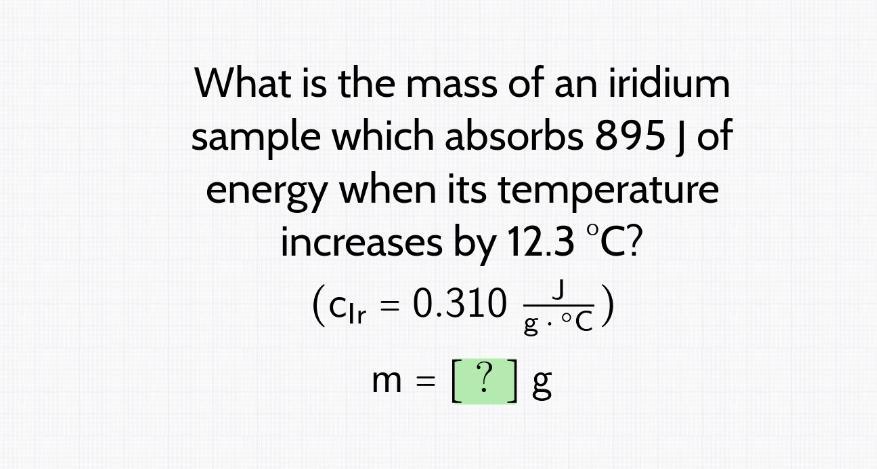
Answers
The mass of an iridium sample which absorbs 895J of energy when its temperature increases by 12.3°C is 234.72g.
How to calculate mass?The mass of a substance can be calculated using the following calorimetry equation:
Q = mc∆T
Where;
Q = quantity of heat absorbed or released (J)m = mass of substancec = specific heat capacity (J/g°C)∆T = temperature (°C)According to this question, an iridium sample absorbs 895J of energy when its temperature increases by 12.3°C. The mass can be calculated as follows:
895 = m × 0.310 × 12.3
895 = 3.813m
m = 234.72g
Therefore, 234.72g is the mass of the iridium sample.
Learn more about mass at: https://brainly.com/question/28992424
#SPJ1
Find the number of grams in 4.26 X 1024 formula units of MgCl2.
Answers
Answer:
Explanation:
Number of moles of Cl atoms in 1.20 1024 Formula units of magnesium chloride mgcl2
The number of moles of Cl is twice as much, because the ratio of Cl in MgCl2 to MgCl2 is 2:1. Therefore, there are 12 moles of Cl. There are 7.22×1024 atoms Cl in 3.61×1024 formula units of MgCl2
The number of grams in 4.26 × 10²⁴ formula units of MgCl₂ is equal to 673.06 grams.
What is the relation between moles and mass?Mass of any substance from moles will be calculated by using the below equation as:
n = W/M, where
W = given mass
M = molar mass
And relation between moles and atoms per moles is:
In 1 moles = 6.022 × 10²³ atoms are present
Given number of atoms of MgCl₂ = 4.26 × 10²⁴
Moles of MgCl₂ = 4.26 × 10²⁴atoms / 6.022 × 10²³ atoms/mol = 7.07 mol
Non we convert this moles of MgCl₂ into grams by using the above given formula as:
W = (7.07mol)(95.2g/mol) = 673.06 g
Hence required mass of MgCl₂ is 673.06g.
To know more about moles & mass, visit the below link:
https://brainly.com/question/15373263
What is the molarity of a solution that contains 152 g NaCl in 4.00 L solution?
Answers
Answer:
200.0lg
Explanation:
please give a brainliest
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
How many particles are in 3.4 moles of NaCl?
Answers
Answer:
The answer is
2.049 × 10²⁴ particlesExplanation:
To find the number of entities or particles given the number of moles of a substance we use the formula
N = n × Lwhere n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question
n = 3.4 mol
We have
N = 3.4 × 6.02 × 10²³
We have the final answer as
2.049 × 10²⁴ particlesHope this helps you
Why don't she love me man
Answers
Answer:
Prob because you ugly as hell or you don't have money.
Explanation:
don't worry about girls man, get your bag up.
What does Chronic Disorder mean?
Answers
Answer:
Chronic diseases are defined broadly as conditions that last 1 year or more and require ongoing medical attention or limit activities of daily living or both. Chronic diseases such as heart disease, cancer, and diabetes
Answer:
conditions that last 1 year or more and require ongoing medical attention or limit activities of daily living
Explanation:
HELPPPPP WILL MARK BRAINLIEST
Which are advantages of reflecting telescopes? Check all that apply.
A)There is no rainbow-like halo around the image.
B)Reflecting telescopes can be made very large.
C)Reflecting telescopes use only lenses, not mirrors.
D)Only the reflecting side of the primary mirror needs to be perfectly shaped and smooth.
E)The eyepiece creates an image for the observer.
Answers
Answer: a.b.d
Explanation:
Answer:
A)There is no rainbow-like halo around the image. Yes
B)Reflecting telescopes can be made very large. Yes
C)Reflecting telescopes use only lenses, not mirrors. No
D)Only the reflecting side of the primary mirror needs to be perfectly shaped and smooth. Yes
E)The eyepiece creates an image for the observer. No
The missing box would be the number _________ and this is a form of
____________ decay.

Answers
Answer: 86,
α-decay
Explanation:
