Answers
The quantum number n, also called 'principal quantum number' refers to the energy level of the electron. It means that we can determine it by the integer value of the expression, like this:
\(\begin{gathered} 3d\text{ }\rightarrow\text{ The principal quantum number \lparen n\rparen is 3.} \\ \text{ }\rightarrow The\text{ azimutal quantum number \lparen l\rparen is d. } \end{gathered}\)So, the answer is A. +3
Related Questions
The chemical potassium sulfate, commonly known as potash of sulfur, is used in fertilizers. This chemical provides both potassium and sulfur to the soil and is easily soluble in water. Potassium sulfate is formed when a strong acid like sulfuric acid (H2SO4) reacts with a strong base like potassium hydroxide. Write the complete balanced molecular equation for this neutralization reaction.
Answers
Answer: \(H_2SO_4(aq)+2KOH(aq)\rightarrow K_2SO_4(aq)+2H_2O(l)\)
Explanation:
Neutralization is a chemical reaction in which acid and base combine to form salt and water. Acids donate \(H^+\) ions and bases donate \(OH^-\) ions in water which combine to form water molecules.
The balanced molecular equation is ;
\(H_2SO_4(aq)+2KOH(aq)\rightarrow K_2SO_4(aq)+2H_2O(l)\)
how many moles of sodium hydroxide are needed to produce 6.0 moles of calcium hydroxide according to the reaction below?
Answers
There would be 12 moles of sodium hydroxide are needed to produce 6.0 moles of calcium hydroxide.
First we should write the balance chemical reaction
CaCl₂ + 2NaOH → Ca(OH)₂ + 2NaCl
From the chemical reaction we know that 1 moles of CaCl₂ react with 2 moles NaOH produced 1 moles of calcium hydroxide and 2 moles of NaCl.
To calculated moles of sodium hydroxide are needed to produce 6.0 moles of calcium hydroxide we can use unitary method
Moles of NaOH = 2/1 x moles of calcium hydroxide
Moles of NaOH = 2/1 x 6 moles
Moles of NaOH = 12 moles
Therefore, to make 6.0 moles of calcium hydroxide, 12 moles of sodium hydroxide would be required.
Your question is incomplete but most probably your full question was
how many moles of sodium hydroxide are needed to produce 6.0 moles of calcium hydroxide according to the reaction below?
CaCl₂ + 2NaOH → Ca(OH)₂ + 2NaCl
Learn more about moles at https://brainly.com/question/27750645
#SPJ4
Explain how to scale a life size crime scene onto a piece of sketch paper
Provide an example with correct calculations
also provide a citation
(ignore my tag where it says chem it's forensics science but ig this site doesn't have this tag)
Answers
Scaling a life-size crime scene onto a sketch paper involves reducing the dimensions of the scene while maintaining accurate proportions.
Here's an example of how to do it:
Measure the dimensions of the crime scene (e.g., length and width) using a tape measure.
Determine the desired scale for the sketch (e.g., 1 inch represents 1 foot).
Calculate the reduction factor by dividing the length of the crime scene by the length on the sketch paper. For example, if the crime scene length is 30 feet and the sketch length is 10 inches (120 inches), the reduction factor would be 30/120 = 0.25.
Multiply all measurements of the crime scene (length, width, objects, distances) by the reduction factor to obtain the corresponding measurements for the sketch.
Transfer the scaled measurements onto the sketch paper using a ruler and appropriate drawing tools.
Citation: The procedure described above is a commonly used method for scaling objects or scenes in forensic science investigations. It is based on principles of measurement and proportion commonly employed in the field. No specific citation is provided since this is a widely used technique in forensic science practice.
Therefore, scaling a life-size crime scene onto a sketch paper involves reducing the dimensions of the scene while maintaining accurate proportions.
for more such question on crime scene
https://brainly.com/question/25759350
#SPJ8
Please help solve these chemistry questions!!!
Practice with percent composition.
1. What is the percent composition of Fe if 111.7 g of Fe is recovered from a 159.7 g sample of Fe2O3?
2. What is the percent composition of O if 12 g of O is recovered from a 38 g sample of Cr2O3?
3. Calculate the percent composition of KCl.
4. Calculate the percent composition of N2O5.
Practice with empirical formulas
5. What is the empirical formula for a compound that contains 0.0260 mol C, 0.0325 mol H, and 0.0065 mol O.
Practice with Molecular Formulas
6. Determine the molecular formula for a compound that has an empirical formula of NO2 and a molar mass of 138.015 g/mol.
7. Nicotine is 74.1% C, 8.6% H, and 17.3% N by mass. It’s molar mass is about 160 g/mol.
a. What is it’s empirical formula?
b. What is it’s molecular formula?
Answers
To calculate the percent composition of Fe:
(55.85 g/mol / 159.7 g/mol) × 100% = 34.9%
Therefore, the percent composition of Fe in Fe2O3 is 34.9%.
Similarly, to find the percent composition of O in Cr2O3, we need to calculate the molar mass of O and the molar mass of Cr2O3. The molar mass of O is 16.00 g/mol, and the molar mass of Cr2O3 is 152 g/mol.
To calculate the percent composition of O:
(16.00 g/mol / 152 g/mol) × 100% = 10.5%
Therefore, the percent composition of O in Cr2O3 is 10.5%.
The formula for potassium chloride (KCl) consists of one potassium atom (K) and one chlorine atom (Cl). To calculate the percent composition of KCl, we need to calculate the molar mass of KCl.
The molar mass of KCl is:
39.10 g/mol (molar mass of K) + 35.45 g/mol (molar mass of Cl) = 74.55 g/mol
To calculate the percent composition of K:
(39.10 g/mol / 74.55 g/mol) × 100% = 52.4%
To calculate the percent composition of Cl:
(35.45 g/mol / 74.55 g/mol) × 100% = 47.6%
Therefore, the percent composition of KCl is 52.4% K and 47.6% Cl.
The formula for dinitrogen pentoxide (N2O5) consists of two nitrogen atoms (N) and five oxygen atoms (O). To calculate the percent composition of N2O5, we need to calculate the molar mass of N2O5.
The molar mass of N2O5 is:
2 × (14.01 g/mol) (molar mass of N) + 5 × (16.00 g/mol) (molar mass of O) = 108.01 g/mol
To calculate the percent composition of N:
(2 × 14.01 g/mol / 108.01 g/mol) × 100% = 25.9%
To calculate the percent composition of O:
(5 × 16.00 g/mol / 108.01 g/mol) × 100% = 74.1%
Therefore, the percent composition of N2O5 is 25.9% N and 74.1% O.
To determine the empirical formula for a compound with given mole ratios, we need to divide each mole value by the smallest mole value to obtain the simplest whole number ratio.
Given:
0.0260 mol C
0.0325 mol H
0.0065 mol O
Dividing each mole value by 0.0065 (the smallest mole value), we get:
C: 0.0260 mol / 0.0065 mol = 4
H: 0.0325 mol / 0.0065 mol = 5
O: 0.0065 mol / 0.0065 mol
What is the vapor pressure of CH3COOH and 90*C?
Answers
Answer:
40.kPa hope this helps....
A sample of a certain lead compound contains 12.92 g of lead for 2 g of oxygen. A second sample has mass of 34.27 g and contains 14.39 g of oxygen. Are the two compound the same
Answers
The two chemical compounds are not the same, because their ratio is not equal. In both samples the composition of lead and oxygen is different.
What is a chemical compound?A chemical compound is a substance made of numerous similar molecules (or molecular entities) joined by chemical bonds and comprising atoms from various chemical elements. Therefore, a molecule made up of only one type of atom is not a compound. Chemical reactions, which may entail interactions with other molecules, can change a compound into a distinct substance. Atomic bonds may be broken or new ones created during this process.
What are the calculations?sample 1 = mass of lead / mass of oxygen = 12.92g/2g = 6.46 .
sample 2 = mass of lead/ mass of oxygen = 34.27 - 14.39/14.39 = 1.38 .
so, the ratios are not the same.
Hence, the two chemical compounds are not the same, because their ratio is not equal. In both samples the composition of lead and oxygen is different.
To know more about Chemical compounds, check out:
https://brainly.com/question/26487468
#SPJ1
What tiles should I put in the box?

Answers
Answer:
true&answe
Explanation:
Question 7
Which of the following would have the longest bond?
ON₂
ON₂²-
OB₂
09₂
Please answer ASAP
Answers
The specie that would have the longest bond is ON₂. Option A
Which bond is the longest?We know that the length of a bond has to do with the order of the bond generally we know that the shorter the bond is, the greater the s character of the bond and the implication of that is that the bond would be much shorter and this would have to come into play here.
We now have to look at each of the bonds and keep the template at the back of our minds that the single bonds are longer than the double bonds and the double bonds are longer than the triple bonds.
In effect, the higher the bond order of the compound, the shorter then bond between the elements that we have in the compound would be from the basics of chemistry. Hence the options would show us that ON₂ has the longest bond here.
Learn more about bond length:https://brainly.com/question/14924352
#SPJ1
How many electrons (total number) are in the ion, CO3(2-) ?
Select one:
a.30
b. 24
c. 32
d. 16
Answers
about how much of the visible side of the moon is lit up during a full moon?
A. Three fourths
B. One fourth
C. None of it
D. All of it
Answers
Answer:
D
Explanation:
i learned this in elementary
You have an aqueous solution and add more and more base to it and plot the pH. You do the same experiment again, but the second time with a buffer in the solution. Compared to the solution without the buffer, for the solution with the buffer, the curve of the pH versus the amount of base added will
Answers
If a strong base is added to a buffer the pH will change only slightly. In the non-buffered solution changing the pH significantly.
If a strong base is added to a buffer, the weak acid will give up its H+ in order to transform the base (OH-) into water (H2O) and the conjugate base: HA + OH- → A- + H2O. Since the added OH- is consumed by this reaction, the pH will change only slightly.
In the non-buffered solution, the added hydronium or hydroxide ions have nothing to react with so the concentrations increase rapidly, changing the pH significantly. If a base is added to an acidic solution, the solution becomes less acidic and moves toward the middle of the pH scale.
Learn more about pH values here:
https://brainly.com/question/15289714
#SPJ4
Volume in ml of 0.220 m hbr solution is required to produce 0.0170 moles
Answers
Molality is defined as the number of moles of solute present in 1L of solution.
Molality (M) = No of moles of solute / 1 L of solution
Litre of solution = No of moles of solute / Molality
Molality = 0.220M
No of moles= 0.0170 mol
Volume in L = 0.0170/0.220
= 0.0772 L
Volume in mL = 77.27 mL
What is mole?
A mole is a very important unit of measurement used by chemists.moles of something means there are 602,214,076,000,000,000,000,000,000,000,000 number of something.Chemists need to measure very small things such as atoms, molecules and other particles in moles.602,214,076,000,000,000,000,000 is called Avogadro's number, abbreviated as 6.02 x \(10^{23}\)Therefore, Volume in ml of 0.220 m hbr solution is required to produce 0.0170 moles is 77.27 mL.
To know more about Molality, visit:
https://brainly.com/question/24065939
#SPJ1
Many gyms and health clubs have steam saunas, which are small steam-filled rooms. Traditionally, steam saunas have a container of heated rocks. A small ladle of water is poured on the rocks in order to make the steam. Use what you have learned so far about heat transfer to explain how hot rocks can be used to make steam.
Answers
Solution :
It is given that a now-a-days many of the health clubs and the gyms provides steam saunas with the help of heated rocks in a container. When water is poured in to these heated rocks, steam is being produce.
This is because the energy conversion takes place in this process. The hot rocks have high temperatures and possess heat energy in them. So when cold water is poured in to the rocks, the cold water absorbs the heat energy from the rocks and is converted in to hot vapor by converting heat energy in to vapor energy or steam energy by the process of vaporization.
how the El Niño event affected the weather, food production, water supply, or human health?
El Niño was responsible for the following events in 2015:
16 tropical cyclones in the central Pacific hurricane basin
three category 4 hurricanes occurred at the same time
emergency water rationed in St. Lucia and San Juan
65 percent of Antigua's farmers went out of business
northern, central, and southeastern Ethiopian highlands received 50–90 percent of their normal rainfal.
Answers
Answer:
EL Niño had hurricanes and so many damages in nature. :)
Explanation:
it creates warm weather, there is low pressure towards canada
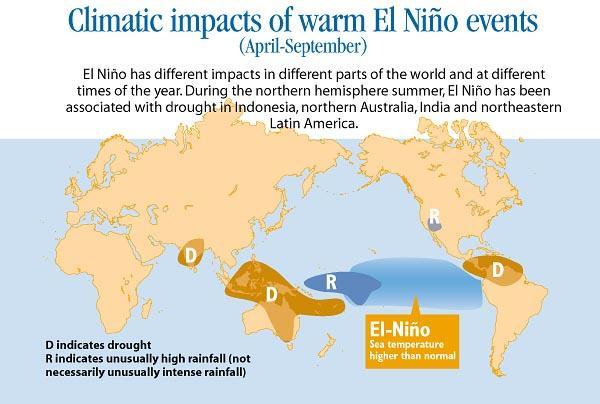
Numerous health issues, such as disease outbreaks, malnutrition, heat stress, and respiratory illnesses are being brought on by the severe drought and associated food insecurity, flooding, precipitation, and temperature increases brought on by El Nino.
What is El- Nino?The exceptional warming of surface waters in the eastern tropical Pacific Ocean is referred to as El Nino, a climate pattern. El Nino is a bigger phenomena known as the El Nino-Southern oscillation, and it is its warm phase.
Climate change brought on by El Nino can be severe and extensive. Increased precipitation results from convection over warmer surface waters. In Ecuador and northern Peru, rainfall rises dramatically, causing coastal erosion and flooding.
Homes, businesses, schools, and hospitals may be destroyed by heavy rains and flooding. They hinder transportation and obliterate crops. As reservoirs dry up and rivers carry less water, the droughts pose a threat to the area's water resources. Agriculture is in danger since it depends on water for irrigation.
Find more on El-Nino:
https://brainly.com/question/18307318
#SPJ2
Please help, its due today! I'll also make you brainiest (put them in an order that's simple, look at the picture and you'll see what I mean) Thank you and God bless! <33
On beaches there are often areas of grassy dunes where people are prohibited from walking. How do these protected areas preserve ecosystem services? Use the graphic organizer to categorize the following as either examples of land reclamation of protecting biodiversity.

Answers
Answer:
Preventing erosion – Land Reclamation
Protecting nesting areas – Protecting Biodiversity
Preventing littering – Land Reclamation
Preventing habitat disruption – Protecting Biodiversity
Protecting native species – Protecting Biodiversity
Preventing contamination of soil – Land Reclamation
Explanation:
I really hope I'm right! I tried my hardest, please give me brainliest :)
have a good day!
lphins... Acid. (b) Chlorine reacts with red hot iron powder to give Iron(III) Chloride but not Iron (II) Chloride. Explain. (1Mark)
Answers
(a) Because acid is caustic, dolphins can perish from exposure to it. Acids are compounds that give other things protons (H+). Acid can react with the proteins and lipids in dolphins' skin when they come into touch with it, leading to chemical burns and damage to the underlying tissue. Systemic consequences from this include death.
(b) Because chlorine is a potent oxidizer, it interacts with red-hot iron powder to produce Iron(III) chloride (FeCl3) rather than Iron(II) chloride (FeCl2). FeCl3 is created when chlorine at high temperatures rapidly accepts electrons from iron atoms. Contrarily, iron interacts with HCl, a less potent oxidizer than chlorine, to produce FeCl2.
Learn more about chlorine at :
https://brainly.com/question/31560014
#SPJ1
Assuming ideal solution behavior, what is the boiling point of a solution of 115.0 g of nonvolatile sucrose (table sugar), C₁₂H₂₂O₁₁ (342.300 g/mol), in 350.0 g of water (Kb = 0.512 °C m⁻¹; boiling point = 100.0 °C)?
a.)
100.00049 °C
b.)
99.5 °C
c.)
268.2 °C
d.)
100.5 °C
Answers
The boiling point of water is 100.0 °C, the boiling point of the solution will be : 101.49 °C.The correct answer is option (a) 100.00049 °C.
Ideal Solution : An ideal solution is a homogeneous mixture of two or more components that obeys Raoult's law, which states that each component's vapor pressure is proportional to its mole fraction.The boiling point of a solution depends on the solvent's properties and the solute's concentration. It's dependent on the mole fraction of the solvent and solute, as well as the total concentration of the solution. The change in boiling point of a solution is given byΔTb = Kb × m × i, whereKb = ebullioscopic constant, m molarity of the solution, and i = van't Hoff factor.Assuming that the solution's behavior is ideal, we may use the molality of the solution to compute the boiling point elevation of the solution.The molality of the solution is given by the following formula:m = (n₂ / m₂) ÷ (n₁ / m₁), where n is the number of moles, m is the mass, and the subscripts 1 and 2 refer to water and non-volatile solute sucrose, respectively.The molar mass of sucrose (C₁₂H₂₂O₁₁) is342.3 g/mol; therefore, the number of moles of sucrose is115.0 g ÷ 342.3 g/mol = 0.335 mol.m₁ = mass of water = 350.0 g, and m₂ = mass of sucrose = 115.0 g, as given in the problem.Therefore, the molality of the solution is given by:m = (0.335 mol / 0.115 kg) ÷ (1 mol / 1 kg) = 2.91 mol/kg.Substituting these values in the formula for ΔTb, we get:ΔTb = Kb × m = 0.512 °C m⁻¹ × 2.91 mol/kg = 1.49 °C.100.0 °C + 1.49 °C = 101.49 °C.
for such more questions on solution
https://brainly.com/question/25326161
#SPJ8
If the atom is assumed to be a sphere, what is the volume in cm^3 of a single Au atom?
Answers
Answer:
The equation for a volume of a sphere is V= (4/3)πr3
The above equations says we need to find the radius of our sphere. According to https://www.ptable.com/ the radius of a gold atom is 144 pm.
The questions wants the answer to be in cm3 which means we need to convert picometers into centimeters. 1cm= 1010pm
144pm x (1cm/1010pm) = 1.44 x 10-8cm
Now we plug the radius of the gold atom into the volume equation.
V= (4/3)π(1.44x 10-8cm)3 -----> Answer= 1.25x10-23 cm3
HOPE IT'S HELPS YOUWhat mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
The formula for density is
Answers
Answer: d=m/v
Explanation: The formula for density is d = M/V, where d is density, M is mass, and V is volume. Density is commonly expressed in units of grams per cubic centimetre.
How does the density of salt water differ from that of freshwater
Answers
Answer:
denser than freshwater due to the sodium chloride dissolved in it.
Explanation:
t. This means that a specific volume of salt water is heaver than the same volume of freshwater. ... While colder water is denser, when water freezes into ice, it becomes less dense and floats on the surface
amobarbital sodium react with ethanolic sodium hyrooxide
Answers
Amobarbital (like all barbiturates) works by being incontestible to the GABAA receptor at either the alpha or the beta subunit.
What is the mechanism of amobarbital?Amobarbital (like all barbiturates) works by binding to the GABAA receptor at either the alpha or the beta subunit. These are compulsory sites that are distinct from GABA itself and also distinct from the benzodiazepine binding site.
Amobarbital is a barbiturate classified as having a halfway duration of action, meaning that the effects of the drug can last from 4-6 amobarbital increases the effects of benazepril by apparatus: pharmacodynamic synergism.
So we can conclude that Amobarbital, 5-ethyl-5-isoamyl barbituric acid like all barbiturates.
Learn more about Amobarbital here: https://brainly.com/question/7237163
#SPJ1
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
The graph shows five data points collected in an investigation of the relationship between the concentration of alcohol dissolved in water and its density. The relationship was expected to be linear. Which of the data points most likely resulted from an error in procedure? a 1 b 2 c 4 d 5
Answers
In comparison to modern, highly accurate density meters or pycnometers, hydrometers are far less accurate and temperature.
Thus, Although they require very large sample sizes, hydrometers are rather simple to operate. Usually, 300 to 500 ml per measurement are required. Hydrometers frequently require calibration off-site as well.
With measurements taken by eye, user error is a major issue, and temperature management is especially challenging. Inaccurately bringing and maintaining samples at temperature might take a long time, and once more, user perception of temperature levels is used to determine temperature levels.
Pycnometers and hydrometers have a further problem in that the findings of alcohol measurement are challenging to evaluate and record.
Thus, In comparison to modern, highly accurate density meters or pycnometers, hydrometers are far less accurate and temperature.
Learn more about Temperature, refer to the link:
https://brainly.com/question/7510619
#SPJ1
Compounds X and Y both have the formula C7H14. Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylhexane. The heat of hydrogenation of X is greater than that of Y. Both X and Y react with HCl to give the same single C7H15Cl compound as the major product. What is the structure of X?
Answers
Answer:
See explanation and image attached
Explanation:
Alkenes undergo hydrogenation to give the corresponding alkanes. Where the structure of the original alkene is unknown, we can deduce the structure of the alkene from the structure of the products obtained when it undergoes various chemical reactions.
Now, the fact that we obtained 2-methylhexane upon hydrogenation and the two compounds had different heats of hydrogenation means that the two compounds were geometric isomers. The original compounds must have been cis-2-methyl-3-hexene and trans-2-methyl-3-hexene.
When reacted with HCl, the same compound C7H15Cl is formed because the stereo chemistry is removed.
However, we know that the trans isomer is more stable than the cis isomer hence the cis isomer always has a higher heat of hydrogenation than the trans isomer. Thus X is cis-2-methyl-3-hexene.

Which type of biochemical is shown in this illustration?
carbohydrate
nucleic acid
lipid
protein

Answers
Option b is the correct answer. Nucleic acid is a type of biochemical is shown in this illustration.
The representation gave is an improved on variant of the design of a nucleotide, which is a structure block of nucleic acids like DNA and RNA. The nucleotide comprises of three parts: a sugar particle (displayed in the representation as CH2OH), a nitrogenous base (not displayed in this delineation), and a phosphate bunch (displayed in the representation as - O-P=O). The phosphate bunch is adversely charged and can shape hydrogen bonds with different nucleotides to make the foundation of the nucleic corrosive strand. The plan of nucleotides and the arrangement of the nitrogenous bases decide the hereditary code and capability of the nucleic corrosive.
To learn more about nucleic acid, refer:
brainly.com/question/30972834
#SPJ1
When fossil fuels are burned, they emit carbon dioxide into the atmosphere. After centuries of large amounts of carbon dioxide accumulating in the atmosphere, the earth's temperature increases by 1°C.
What is the connection between increasing carbon dioxide and increasing temperature?

Answers
The connection between increasing carbon dioxide and increasing temperature is: carbon dioxide absorbs heat from the sun and traps it in earth's atmosphere. Since the heat cannot escape, it causes the earth's temperature to increase which is the first option.
When carbon dioxide (CO₂) and other greenhouse gases are present in the atmosphere, they act as a natural blanket, allowing sunlight (solar radiation) to pass through and reach the Earth's surface. Some of this solar radiation is absorbed by the Earth's surface, while the rest is reflected back towards space as heat (infrared radiation). However, greenhouse gases like carbon dioxide have the property of absorbing and re-emitting infrared radiation.
Learn more about fossil fuel here
https://brainly.com/question/2029072
#SPJ1
A sample of xenon gas occupies a volume of 5.82 L at 453 K. If the pressure remains constant, at what temperature will this same xenon gas sample have a volume of 2.64 L?
Answers
Answer:
204.8 K
Explanation:
(P1 * V1)/T1 = (P2 * V2)/T2
"If the pressure remains constant" means
you can cancel the pressure part of the equation
T2 = (V2 * T1)/(V1)
T2 = (2.64 L * 453 K)/(5.82 L)
T2 = 204.8 K
chatgpt
A sample of xenon gas occupies a volume of 5.82 L at 453 K. If the pressure remains constant, the temperature that will make this same xenon gas sample have a volume of 2.64 L is 220K.
What is combined gas law?The combined gas law is the law of of gaseous state which is made by combination of Boyle's law, Charle's law, Avogadro's law and Gay Lussac's law.
It is a mathematical expression that relates Pressure, Volume and Temperature.
(P1 × V1)÷T1 = (P2 × V2)÷T2
We can also use the following relation-
PV = nRT
At constant pressure,
V1÷T1 = V2÷T2
This is Charles's law.
V1 = 5.82L
T1 = 453K
V2 = 2.64L
T2 = ?
T2 = 220K
Therefore, A sample of xenon gas occupies a volume of 5.82 L at 453 K. If the pressure remains constant, the temperature that will make this same xenon gas sample have a volume of 2.64 L is 220K.
Learn more about combined gas law, here:
https://brainly.com/question/30458409
#SPJ2
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
If 128g of a certain gas in a container with a volume of 21.5 L has a pressure of 140 kPa and a temperature of 45°C, what is the molar mass of the gas?
show how you get the answer please
Answers
If 128g of a certain gas in a container with a volume of 21.5 L has a pressure of 140 kPa and a temperature of 45°C, the molar mass of the gas is 112 g/mol
The Ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. The ideal gas equation can be written as
PV = nRT
where,
P = Pressure
V = Volume
T = Temperature
n = number of moles
Given,
Volume = 21.5L
Pressure = 140 kPa
Temperature = 318 K
Using ideal gas equation, PV = nRT
140 × 21.5 = n × 8.314 × 318
n = 1.138 moles
Mass = moles × molar mass
Molar mass = 128 ÷ 1.138
= 112 g/mol
Learn more about Ideal Gas Equation, here:
https://brainly.com/question/28837405
#SPJ1