Answers
a) Carbon tetra chloride in the preparation of alkynes acts a solvent
b) Hydrogen bromide in the preparation of alkynes adds across the multiple bonds of the alkyne
What is the use of the reagents?We know that in a chemical reaction, there are certain chemical species that are involved in the reaction because they interact and lead to the final products. In effect, the discussion about a chemical change just have to do with how reactants are able to interact with each other, bonds are broken and new bonds are made.
In each of the cases that have been mentioned in the question, what we have is an addition reaction. We can see that there is a substrate that has some reagents that act on it and then there is the product that we get as the bonds in the substrate are broken and new bonds are made.
Learn more about addition reaction:https://brainly.com/question/13669873
#SPJ1
Related Questions
Arrange the following three ionic compounds in the order of increasing lattice energy (from smallest lattice energy to largest lattice energy).
MgBr₂
MgO
KBr
Answers
The expected lattice energies, in increasing order, are MgO> MgBr2> KBr.
The ranking is related that the lattice energy being directly proportional to the strength of the ionic bond. If the bond is strong, the lattice energy would be high.
If the intermolecular force is strong, then the energy requires to break the bond would be greater. Thus, the lattice energy would be high.
MgO has the lowest lattice energy because it is the smallest and has the weakest attractive force between the ions. MgBr2 has a slightly higher lattice energy because the bromine ions are larger and have a stronger attractive force than the oxygen ions. KBr has the highest lattice energy because the potassium ions are larger than the bromine ions and have a stronger attractive force.
To know more about ionic bonds, click below:
https://brainly.com/question/2220825
#SPJ1
1. What was the immediate cause for the United States' entry into World
War II?
O The attack on Pearl Harbor by Japan
O The German invasion of Poland
O The formation of the Axis powers
O The rise to power of Adolf Hitler
Answers
The immediate cause for the United States' entry into World War II was the attack on Pearl Harbor by Japan on December 7, 1941.
What was World War II about?World War II was a global war that lasted from 1939 to 1945. It involved the majority of the world's nations, including all of the great powers, organized into two opposing military alliances: the Allies and the Axis.
Prior to this attack, the United States had maintained a policy of neutrality and had provided support to the Allies in the form of aid and supplies. However, the attack on Pearl Harbor, which resulted in the deaths of over 2,400 Americans, led to a declaration of war against Japan by the United States, and ultimately to the country's involvement in the larger global conflict.
Learn more on World War II here: https://brainly.com/question/27005787
#SPJ1
What element does “X” represent in Figure 1?
Helium
Krypton
Neon
Argon

Answers
Answer:
Argon
Explanation:
It's atomic number is 18
hope that's it
Consider the following equilibrium:
HC2O4- + HSO4- <—> H2C2O4 + SO42-
The order of Bronsted-Lowry acids and bases in the reaction is_________ .
Answers
Answer:
See explanation
Explanation:
According to Bronsted-Lowry definition of acids/bases, an acid is a proton donor while a base is a proton acceptor.
Hence, in a reaction, the species that donate protons are strong acids while the species that accept protons are strong bases.
Hence, HSO4- is a stronger acid than H2C2O4 and SO42- is a weaker base than HC2O4-
Note that the conjugate base of a strong acid is a weak base and vice versa.
x gm of metal (E=12) was completely dissolved in 100 ml of N/2 HCl. The solution was then made upto 500 ml. 2.5 ml of this diluted acid required 12.5 ml of N/10 NaOH for complete neutralization. Find the valency of x.
Answers
When a potent acid, like hydrochloric acid, HCl, is combined with a potent base, such sodium hydroxide, NaOH, the result can be complete neutralization. Strong bases and acids dissociate entirely into their individual ions when they dissolve in water.
An element's valency is its capacity for combination. The periodic table groups have identical elements with the same valency.. How many electrons make up the outer shell of an element determines its valency. The group number.
Valency is simply the amount of electrons that an element's atom gains, loses, or shares to obtain the closest configuration to that of a noble gas. The valency of sodium (Na), magnesium (Mg), chlorine (Cl), and other elements, for instance, is 1.
When there are equal moles of strong acid and strong base, the strong acid and strong base completely neutralize one another. An aqueous solution with a pH of 7 is the end product.
To know more about valency, click on the link below:
https://brainly.com/question/24880893
#SPJ9
Gaseous ammonia chemically reacts with oxygen O2 gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of water produced by the reaction of 0.070mol of ammonia. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
Answers
The moles of the water produced by the reaction of 0.070 mol of ammonia is the 0.175 mol.
The reaction is given as:
4NH₃(g) + 5O₂(g) ---> 4NO(g) + 5H₂O(g)
The moles of ammonia, NH₃ = 0.070 mol
The moles if substances can be calculated as :
The number of moles = mass / molar mass
The 4 moles of the NH₃ will produces the 4 moles of NO
4 moles of ammonia, NH₃ produces the 5 moles of water , H₂O
The moles of water, H₂O = ( 5 / 2 ) × 0.070 mol
= 0.175 mol
Thus, the moles of water, H₂O is 0.175 mol.
To learn more about moles here
https://brainly.com/question/15994622
#SPJ4
Categorize each molecule according to its hydrogen-bonding characteristics.a. CH3OCH3b. CH3CH2CH3c. CH3CH2NH2d. H2Oe. CH3OH
Answers
Answer:
H2O > CH3OH > CH3CH2NH2 > CH3OCH3 > CH3CH2CH3
Explanation:
Hydrogen bonding exists in molecules in which hydrogen is bonded to a highly electronegative element such as fluorine, chlorine, oxygen, sulphur, nitrogen etc.
Water has the highest degree of hydrogen bonding among the listed substances hence its very high boiling point, stemming from intermolecular hydrogen bonding. CH3CH2NH2 has a lesser degree of hydrogen bonding because nitrogen is less electronegative than oxygen.
Alcohols show significant hydrogen bonding in solution, the same applied to amines. However, ethers do not form hydrogen bonds with each other but they can form hydrogen bonds with other molecules such as alcohols and amines. Alkanes do not form hydrogen bonds at all.
Suppose a student is given 4.123 g
of a powered mixture to separate which contains iron shavings. The iron (Fe)
is removed from the mixture using a magnet and weighed on a balance. The mass of the iron is 1.213 g.
What is the mass percent of iron in the mixture?
Answers
The mass percent of iron in the mixture is 29.43%.
What is mass percent?
Mass percent, also known as percent by mass, is a way of expressing the concentration of a solute in a solution, or the composition of a mixture, in terms of the mass of the solute or component relative to the total mass of the solution or mixture, multiplied by 100%.
To determine the mass percent of iron in the mixture, we need to use the formula:
mass percent = (mass of iron / total mass of mixture) x 100%
We are given that the mass of the iron is 1.213 g, and the total mass of the mixture is 4.123 g.
So, substituting these values into the formula:
mass percent = (1.213 g / 4.123 g) x 100%
mass percent = 29.43%
Therefore, the mass percent of iron in the mixture is 29.43%.
What is concentration of solution?
Concentration of a solution is the amount of solute dissolved in a given amount of solvent, and is usually expressed as a unit of measure per volume or mass of the solution. The most commonly used units of concentration include molarity, molality, mass percent, volume percent, and parts per million.
To know more about mass percent, visit:
https://brainly.com/question/5394922
#SPJ1
A 70% nitric acid solution contains 70g of acid in 100g of solution, i.e. 70g of acid for 30g of water M(HNO3)=
Answers
Given that the nitric acid solution contains 70% nitric acid, this means that for every 100g of the solution, there are 70g of nitric acid. Hence, the remaining 30g is water.Since the question requires the molarity of the solution, we need to first calculate the number of moles of nitric acid in the solution.
Number of moles of nitric acid = Mass of nitric acid / Molar mass of nitric acid Molar mass of nitric acid, HNO3 = 1(atomic mass of hydrogen) + 14(atomic mass of nitrogen) + 3(atomic mass of oxygen) = 63 g/mol For the given solution,Mass of nitric acid = 70g Number of moles of nitric acid = 70/63 = 1.1111 mol Now, we need to calculate the volume of the solution containing 1.1111 mol of nitric acid.
Assuming the density of the solution to be 1 g/mL, the volume of the solution containing 1.1111 mol of nitric acid is given by,Volume of solution = 1.1111 mol / 1 g/mL = 1.1111 L Thus, the molarity of the nitric acid solution is: Molarity, M = Number of moles / Volume of solution M(HNO3) = 1.1111 mol / 1.1111 L = 1 M Ans: M(HNO3) = 1 M
For more such questions on nitric acid
https://brainly.com/question/22698468
#SPJ8
How do you identify a redox reaction?
Answers
Answer:
I think you have to calculate the oxidation number of each atom in the reaction.
Explanation:
Hope that helped! ^u^
Why was meteorology such a late developer compared to other branches of science?
Answers
Answer:
Because of the difficulties of measuring the atmosphere's properties above the earth's reachable surface
Explanation:
Hello,
In this case, meteorology is the branch of science studying the atmosphere in its weather processes and forecasting and it had a late development because of the difficulties of measuring the atmosphere's properties above the earth's reachable surface. We cannot forget that even nowadays, it is very difficult to predict upcoming weathers with the 100 % assurance and with many days in advance.
Best regards.
Meteorology is developed lately as compared to other branches of science due to far away from the reach of humans.
Meteorology is a late developer compared to other branches of science because the measuring the climatic conditions in the atmosphere is difficult and even impossible without the presence of advance technologies.
To find out the weather as well as climatic conditions can't be measured due to it is not reachable to the human like other materials present on the earth surface so we can conclude that meteorology is developed lately as compared to other branches of science due to far away from the reach of humans.
Learn more: https://brainly.com/question/16565664
2. What is an element?

Answers
Answer: the answer is c i looked it up please give me brainliest pleaseeee
Explanation:
A 100 g block of a substance requires 0.5 kJ of heat to raise its temperature from 25.0°C to 37.8°C.
What is the substance?
A) iron
B) aluminum
C) gold
D) copper
Answers
The metal can be identified using its specific heat capacity. Here, the specific heat capacity of the substance calculated from calorimetric equation is 0.39 J/g °C , that is the specific heat of copper metal. Hence, option D is correct.
What is specific heat capacity ?The specific heat capacity of a substance is the heat energy required to raise the temperature of the substance by one degree Celsius per on gram. It is characteristic to the substance.
The calorimetric equation connecting the heat energy q, with mass of the substance m, specific heat c and temperature difference ΔT is given below:
q = m c ΔT.
Given, m = 100 g
q = 0.5 KJ = 500 J
ΔT = 37.8 - 25 °C = 12.8 °C
then c = q/mΔT
c = 500 J/12.8 °C × 100 g = 0.39 J/g °C
this specific heat corresponds to the copper metal.
Therefore, the block is made of copper metal.
Find more on specific heat:
https://brainly.com/question/11297584
#SPJ2
write the atomicity of oxygen
Answers
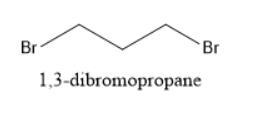
When Ethyl Acetoacetate is treated with 1,3-Dibromopropane and 2 moles of Sodium Ethoxide in Ethanol, a compound is produced that has the formula C9H14O3. This compound has an infrared spectrum that shows only one carbonyl adsorption and no OH bond stretch. Suggest a structure for this compound, and provide a mechanism for its formation.
Answers
Answer:
The reaction of ethyl acetoacetate with 1,3-Dibromopropane and 2 moles of Sodium Ethoxide in Ethanol.
Explanation:
The structure of ethyl acetoacetate is shown below:
The structure of 1,3-dibromopropane is shown below:
Ethyl acetoacetate on reaction with sodium ethoxide in ethanol forms a carbanion intermediate and the formation of carbanion takes place twice and form a cyclic ring.
The entire reaction is shown below:
The structure of C9H14O3 is shown in the chemical reaction that is shown below:


Who made the first periodic table
Answers
The correct answer is Dmitri Mendeleev
You have 3.00 L of a 3.12 M solution of NaCl(aq) called solution A. You also have 2.00 L of a 2.00 M solution of AgNO3(aq) called solution B. You mix these solutions together, making solution C.
Calculate the concentration (in M) of Ag+ ions in solution C.
Answers
Answer:
\(M=0.8M\)
Explanation:
Hello!
In this case, since the presence of silver ions is in AgNO3 only, we can compute their moles by using the volume and concentration of the corresponding salt:
\(n_{Ag^+}=2.00L*2.00\frac{mol}{L}=4molAg^+\)
Next, since the total volume of solution C is 5.00 L, the required concentration of silver ions turn out:
\(M=\frac{4.00mol}{5L} =0.8M\)
Best regards!
If you know the answer tell me please

Answers
Metamorphic rocks can be harder, less porous, and have crystals that can be lined, describing some of the ways in which metamorphic rocks differ from sedimentary rocks.
There are two different types of rocks: sedimentary rocks and metamorphic rocks. Igneous or sedimentary pre-existing rocks undergo changes under extreme heat and pressure to form metamorphic rocks. This process results in the recrystallization of minerals, leading to the formation of a new rock with distinct physical and chemical characteristics.
Therefore, the correct option is B.
Learn more about Metamorphic rocks, here:
https://brainly.com/question/19930528
#SPJ1
Predict the missing component in the nuclear equation.

Answers
Answer:
The first option A (one electron)
Explanation:
From the left side of the arrow, Fr has a mass number of 223 and an atomic number of 87, so we want both of the numbers to be the same on the right side.
On the right side, Rn has a mass number of 223, which is the same on the left side for Fn, so we do not need to add any more to the mass number, hence 0.
Rn has an atomic number of 86, but Fr has an atomic number of 87, so we need one more electron to make both sides equal.
Fluoride is often added to water as sodium fluoride (NaF). What is the mass percent composition of in NaF? How many grams of NaF must be added to 1500 L of water to fluoridate it at a level of 0.7 mg
Answers
The Mass percent composition of F - in NaF is 45.25% .
2.323 g must be added to 1500 L of water to fluoridate it at a level of 0.7 mg.
What is mass percent composition?Mass percent composition describes the relative quantities of elements in a chemical compound.
The given data is:
Volume of water = 1500 L
Amount of F- ion = 0.7 mg/L
Atomic weight of F = 19 g/mol
Number of moles of F- in 1 L of water = 0.7 x 10⁻³ g/19 g mol⁻¹ = 3.684x 10⁻⁵ moles
Therefore, the number of moles of F- in 1500 L of water = 3.684 x 10⁻⁵ x 1500 = 0.0553 moles
1 mole of NaF has 1 mole of Na+ and 1 mole of F-
hence, the number moles of NaF required = 0.0553 moles
Molar mass of NaF = 23 +19 = 42 g/mol
Mass of NaF required = 0.0553 moles x 42 g/mol = 2.323 g
Learn more about Mass percent composition at: https://brainly.com/question/26150306
#SPJ1
You complete titration of 15.0ml of unknown acid with 14.16ml of 1.25 M NaOH. What’s the molar it’s of the unknown acid? Assume the acid is monoprotic 1 mole of acid reacts with 1 mole of base.
Answers
The molarity of the unknown acid : 1.18 M
Further explanationTitration is a procedure for determining the concentration of a solution by reacting with another solution that is known to be concentrated (usually a standard solution). Determination of the endpoint/equivalence point of the reaction can use indicators according to the appropriate pH range
Titration formula
M₁V₁n₁=M₂V₂n₂
monoprotic acid⇒n₁=1
NaOH monoprotic base⇒n₂=1
\(\tt M_1\times 15=1.25\times 14.16\\\\M_1=1.18~M\)
Draw the structure of the product formed when the given compound is heated in aqueous base. The formula for the product is C8H12O. The starting material is a 6 carbon chain where carbon 1 is an aldehyde, carbon 2 has two methyl substituents and carbon 4 is double bonded to an oxygen. THis reacts with N a O H, water and heat.
Answers
Answer:
Please find the graph file in the attachment file.
Explanation:

What happens to the electrons when an electric field is applied?
Answers
When electric voltage is applied, an electric field within the metal triggers the movement of the electrons, making them shift from one end to another end of the conductor
Explanation:
When electric voltage is applied, an electric field within the metal triggers the movement of the electrons, making them shift from one end to another end of the conductor
I need help I don’t understand this is hitting

Answers
Reagents that are entirely consumed by a chemical reaction are known as limiting reagents.
Thus, They are additionally known as limiting reactants or limiting agents. A predetermined quantity of reactants are necessary for the reaction to be completed, according to the stoichiometry of chemical reactions.
In the aforementioned reaction, 2 moles of ammonia are created when 3 moles of hydrogen gas react with 1 mole of nitrogen gas.
In most cases, this reactant dictates when the reaction will end. The reaction stoichiometry can be used to determine the precise quantity of reactant that will be required to react with another element. The limiting agent is determined by the mole ratio rather than the mass of the reactants.
Thus, Reagents that are entirely consumed by a chemical reaction are known as limiting reagents.
Learn more about Chemical reaction, refer to the link:
https://brainly.com/question/22817140
#SPJ1
You are out hiking on a cold snowy day. You put on your battery-heated socks. In which direction is the thermal energy flowing?
There is no thermal energy in this scenario.
Thermal energy is moving from the air to your socks
Thermal energy is moving from your feet to your socks
Thermal energy is moving from your socks to your feet
Answers
The correct answer is that thermal energy is moving from your feet to your socks. The battery-heated socks work by using the heat generated by your body to warm your feet in cold weather.
The body produces heat, which is converted into thermal energy, and moves from your feet to the socks.
In order to warm your feet and make them more comfortable in cold weather, the socks use thermal energy. The thermal energy is only transferred in one direction, from your feet to the socks.
No more energy is produced because the battery-heated socks utilise your body's thermal energy to keep your feet warm.
To learn more about thermal energy visit:
https://brainly.com/question/19666326
#SPJ1
Numbers placed in front of formulas in an aquation are called
Answers
Q25 Apply the Criss-Cross method to determine formulae of the corresponding salt's with
two different examples?
Answers
Answer:
CaCl2
CaO
Explanation:
Ca^2+ 2Cl^-
Ca^2+ O^2-
How many mL of water would you add to 20mL of a 7.5M solution of Hydrochloric Acid in order to have a 2M solution?
Answers
Explanation:
When working with dillutions we usually use this formula.
Vd * Md = Vc * Mc
Where Vc and Mc are the volume and molarity of the concentrated solution and Vd and Md are the volume and molarity of the dilluted solution.
Vd = ? Md = 2 M Vc = 20 mL Mc = 7.5 M
With that formula we can find the volume of the dilluted solution that we can prepare from a determined volume of a concentrated solution.
Vd * Md = Vc * Mc
Vd = Vc * Mc/Md
Vd = 20 mL * 7.5 M/(2 M)
Vd = 75 mL
With the concentrated solution we can prepare 75 mL of the 2 M solution. To prepare that solution we will add some mL of water to the concentrated solution that already had a volume of 20 mL.
Vdilluted = Vwater + Vconcentrated
Vwater = Vd - Vc
Vwater = 75 mL - 20 mL
Vwater = 55 mL
Answer: we have to add 55 mL of water to the 7.5 M solution.
glucose is a six carbon sugar. Albumin is a protein with 607 amino acids. the average molecular weight of a single amino acid is 135 g/mol. there is no reason to run these solutes at the 20 MWCO because
Answers
There is no reason to run these solutes at the 20 MWCO because they are both much smaller than the MWCO of the membrane.
The MWCO (molecular weight cut off) is the molecular weight of a solute at which it will be retained by a membrane during a process such as ultrafiltration or dialysis. If a solute has a molecular weight higher than the MWCO of a membrane, it will be retained and not pass through the membrane. If the molecular weight of a solute is lower than the MWCO, it will pass through the membrane.
In this case, glucose has a molecular weight of 180 g/mol (6 carbons x 12 g/mol per carbon + 6 oxygens x 16 g/mol per oxygen) and albumin has a molecular weight of approximately 81,942 g/mol (607 amino acids x 135 g/mol per amino acid). Both of these solutes have molecular weights that are much lower than 20,000 g/mol, which is a typical MWCO for ultrafiltration or dialysis membranes.
They would both easily pass through the membrane and be lost during the process. Instead, a membrane with a much lower MWCO would be needed if we wanted to retain these solutes during a process such as ultrafiltration or dialysis.
Learn more about glucose here:
https://brainly.com/question/2396657
#SPJ1
The combustion of ethane (C2H6) produces carbon dioxide and steam. 2C2H6(g)+7O2(g)⟶4CO2(g)+6H2O(g) How many moles of CO2 are produced when 5.90 mol of ethane is burned in an excess of oxygen?
Answers
Answer:
11.8 moles of CO2.
Explanation:
We'll begin by writing the balanced equation for the reaction. This is given below:
2C2H6 + 7O2 —> 4CO2 + 6H2O
From the balanced equation above,
2 moles of C2H6 reacted with 7 moles of O2 to produce 4 moles of CO2 and 6 moles of H2O.
Thus, we can obtain the number of moles of CO2 produced when 5.9 moles of C2H6 is burned in excess O2 as follow:
From the balanced equation above,
2 moles of C2H6 reacted to produce 4 moles of CO2.
Therefore, 5.9 moles of C2H6 will react to produce = (5.9 × 4)/2 = 11.8 moles of CO2.
Thus, 11.8 moles of CO2 were obtained from the reaction.