What is the term for the distance that a point on a wave travels during a
specific period of time?
A. Speed
B. Period
C. Amplitude
D. Frequency
Answers
Answer:
It's: Speed!
Explanation:

Related Questions
Do the ingredients changing into a cake in the oven a chemical reaction?
Answers
Answer:
When you bake a cake, the ingredients go through a chemical change. A chemical change occurs when the molecules that compose two or more substances are rearranged to form a new substance! When you start baking, you have a mixture of ingredients. The flour, egg, sugar,
Explanation:
is a single cell the smallest structure that carries out our activities nesscesary for life
Answers
yep, the cell is the smallest unit in a living organism that is capable of carrying out all of the activities of life.
7. A speaker carries a current of 6.4 A when connected to a 120 V source. What is the power output of the speaker? O 1207 W O 982 W O 768 W O 495 W
Answers
The power output of the circuit is the product of the voltage and current passing through the circuit. Hence the power of the speaker carrying a current of 6.4 A when connected to 120 V is 768 W.
What is power?Power is the rate of work done. Its unit is Watt. Power of a device is directly proportional to the current, voltage as well as to the resistance.
The expression relating the power, current and resistance is:
P = I² R
According to Ohm's law, V = I R
Thus, P = I V.
Given that, voltage V = 120 V
current I = 6.4 A.
Thus power P = 120 V × 6.4 A = 768 W.
Therefore, the power of the speaker carrying a current of 6.4 A when it is connected to 120 V source is 768 Watt.
To find more on power, refer here:
https://brainly.com/question/9338858
#SPJ1
How many protons and electrons does a neutral atom of beryllium have
A nine protons,nine electrons
B two protons,two electrons
C four protons,four electrons
D Four proton,five electrons
Answers
Answer:
c
Explanation:
the atomic number is 4 thus beryllium contains 4 protons and 4 electrons
How many isomers are there in C7H16 ?
a. 6
b. 7
c. 8
d. 9
Answers
rx: 0.7 l of 8% omeprazole suspension. your pharmacy stocks: 35% omeprazole suspension. how many ml of the 35% suspension would be needed for the dilution? (round to the nearest hundredth with no units!)
Answers
If the pharmacy stocks 35% omeprazole suspension, it required 160 ml of the 35% omeprazole suspension for the dilution.
It is required to apply the idea of dilution equations to determine the quantity of 35% omeprazole suspension required for dilution.
Let C₁ be the concentration of the 8% omeprazole suspension (8%), and let V₁ be the volume of the 0.7 L 8% omeprazole suspension.
Let C₂ be the concentration of the 35% omeprazole suspension, and let V₂ be the volume of the 35% omeprazole suspension that we need to find.
The dilution equation states that the product of the starting volume and concentration (V₁ × C₁) and the end volume and concentration (V₂ × C₂) should be identical.
V₁ × C₁ = V₂ × C₂
Putting the given values:
0.7 L × 8% = V₂ × 35%
0.056 L = V₂ × 35%
Dividing both sides by 0.35), get:
V₂ = 0.056 L / 0.35
V₂ = 0.16 L
Change 0.16 L to milliliters (ml):
0.16 L × 1000 ml/L = 160 ml
Thus, 160 ml of the 35% omeprazole suspension would be required for the dilution.
Learn more about dilution, here:
https://brainly.com/question/28202548
#SPJ4
how much does 4.2 moles of Ca(NO3)2 weigh?
Answers
Answer:
There are 689.15 grams in 4.2 moles of Ca(NO₃)₂. This can be done by the conversion of moles to grams.
Explanation:
For a detailed step-by-step method on how to solve this, read the Expert-verified answer for this question: https://brainly.ph/question/1303864
draw the lewis structure for the ionic compound that forms from mg and f.
Answers
The Lewis structure for the ionic compound formed from Mg and F is Mg^2+ + 2F^-.
which of the following is a fomite?group of answer choicespuswaterinsectsa hypodermic needledroplets from a sneeze
Answers
A fomite is an object or surface that can carry and transmit infectious microorganisms. Out of the options provided, a hypodermic needle can act as a fomite if it has been contaminated with an infectious agent and is not properly sterilized before reuse. It can spread the infectious agent to other individuals who use the same needle.
It is important to properly dispose of needles and other medical instruments to prevent the spread of infections.
The fomite among the group of answer choices you provided is a hypodermic needle. A fomite refers to any inanimate object or substance that can carry and transmit infectious agents, such as viruses or bacteria.
In this case, a hypodermic needle can potentially carry and transmit infections if not handled or disposed of properly. Other options, such as pus, water, insects, and droplets from a sneeze, are not fomites but rather sources or vehicles of transmission for infectious agents.
To know more about sneeze visit-
https://brainly.com/question/30876748
#SPJ11
A fomite is an object or surface that can carry and transmit infectious agents. Out of the options given, a hypodermic needle is considered a fomite as it can potentially transmit infections if not properly sterilized.
the correct choice among the given options is a hypodermic needle. A fomite is an inanimate object that, when contaminated with infectious agents, can transfer disease to a new host. Pus, water, insects, and droplets from a sneeze are not fomites, as they are either not inanimate objects or they do not typically transfer diseases as objects.
To know more about Fomite visit-
https://brainly.com/question/30455325
#SPJ11
The measurement 0.01011 km has how many significant figures?
Answers
Answer: 4
Explanation:
Sig Figs
4
Decimals
5
Scientific Notation
1.011 × 10-2
Words
zero point zero one zero one one
a chemical company makes a silver by reacting silver nitrate would see the company needs to make 800 g of pure silver for a client they have 300 g of zinc and 600 g of silver nitrate will they be able to make enough silver to fill the order
Answers
Answer
Explanation
Given that:
The mass of pure silver needed = 800 g
Mass of zinc = 300 g
Mass of silver nitrate = 600 g
What to find:
Will the mass of zinc and silver nitrate be able to make 800 g of pure silver.
Step-by-step solution:
Step 1: Write the balanced equation for the reaction.
Zn + 2AgNO₃ → 2Ag + Zn(NO₃)₂
Step 2: Determine the moles of the reactants.
Using the mole formula, the moles of the reactants will be:
\(\begin{gathered} Moles\text{ }of\text{ }Zn=\frac{Mass}{Molar\text{ }mass}=\frac{300g}{65.38g\text{/}mol}=4.5886\text{ }mol \\ \\ Moles\text{ }of\text{ }AgNO_3=\frac{600g}{169.87g\text{/}mol}=3.5321\text{ }mol \end{gathered}\)Step 3: Determine the moles of pure silver produced.
Using the mole ratio of Zn to AgNO₃ in the equation and the moles in step 2, we
PLEASE ANSWER NOW!!!!! WILL GIVE BRAINLIEST!!!
How can you measure the volume of a solid that does not have a regular shape? What property must the solid NOT have for your method to work?
Answers
Answer:
a
b
Explanation:
1 point
When substances that make up baking powder are mixed with water,
carbon dioxide gas is formed. This is an example of *
a nuclear reaction
a physical reaction
a change of state
a chemical reaction
Answers
How does electronegativity change as principal energy levels are added to an atom?
A. Electronegativity decreases.
B. Electronegativity does not change.
C. Electronegativity increases.
D. Electronegativity can increase or decrease; it cannot be known.
+ Answer needed ASAP. will mark as brainliest.
Answers
Answer:A
Explanation: Since more energy levels are added, the pull decreases which means the electronegativity from electrons also decrease.
The electronegativity change as principal energy levels are added to an atom is electronegativity decreases. The correct option is A.
What is electronegativity?The ability of an atom to draw shared electrons in a covalent connection is referred to as electronegativity. The stronger an element attracts the shared electrons, the higher its degree of electronegativity.
As a result, the electronegativity of an element drops as you advance down a group on the periodic table because the larger number of energy levels distances the outside electrons from the nucleus' pull. On the periodic table, electronegativity rises from left to right across each period.
Thus, the correct option is A. Electronegativity decreases regarding the electronegativity decreases with energy levels added.
To learn more about electronegativity, refer to the link:
https://brainly.com/question/17762711
#SPJ2
how many total atoms of each element are presented in the following formula

Answers
Answer:
Aluminium (Al): (3*2)+(5*2)=16
Sulphor (S): (3*1)=3
Oxygen (O): (4*3)+(3*1)=15
please help quickly the answer on the pic I attached

Answers
Answer:
letter C is a structure most like that of slate
Describe how methane is
formed in terms of chemical bonding
Answers
Convert to grams
0.989 moles of phosphorus
1.21 moles of carbon dioxide
Answers
The conversion of the following moles to grams is as follows:
30.659g of phosphorus53.24g of carbondioxideHow to convert moles to mass?The number of moles of a substance can be converted to mass using the following formula:
moles = mass ÷ molar mass
According to this question, 0.989 moles of phosphorus and 1.21 moles of carbon dioxide is given. The mass can be calculated as follows:
Molar mass of P = 31g/molMolar mass or carbondioxide = 44g/molmass of phosphorus = 31 × 0.989 = 30.659g
mass of carbondioxide = 44 × 1.21 = 53.24g
Learn more about mass at: https://brainly.com/question/21042927
#SPJ1
Is the following equation balanced or unbalanced?
H2 + O2 + H2O
Answers
Answer:
its looks unbalanced too me
Explanation:

Which of the definitions below is best for the word molecule? Atoms of different elements chemically joined together More than one atom chemically joined together A type of compound More than one atom of the same type chemically joined together A group of atoms together
Answers
Answer: More than one atom of the same type chemically joined together
Explanation:
An atom is the smallest unit of any matter which may or may not have independent existence. Example: Argon (Ar) is an element which exist as Ar atom only.
Molecule is the smallest unit of any matter which always have independent existence. For example: Hydrogen (H) is an element which can exist in nature as \(H_2\) molecule only.
Atoms of different elements chemically joined together is called as a compound. Example: \(H_2O\) is a compound formed by chemical combination of hydrogen (H) and oxygen (O) atom.
please help there are 3 answers brainlies is included with the best answer.
SUMMATIVE FOSSILS/FOSSIL RECORD/HISTORY OF LIFE ON EARTH
FOSSILS CAN BE CATEGORIZED INTO 3 MAJOR TYPES. THEY ARE:
REPLACEMENT
METAMORPHIC
TRACE
SEDIMENTARY
IMPRESSION
Answers
Answer:
Your answers would be:
-impression fossils
-trace fossils
-replacement fossils
Explanation:
Have a great rest of your day
#TheWizzer

Answer:
The are id-k- I dont know much on fossials. But did you know that thunder is lighting. I say this because thunder is just the noise lighting makes. We see the light firts because light travels faster than sound.
Explanation:
Hope This Helps
Have A Great Day
~Zero~
What should you do with leftover reagents after an experiment? What should you do with the products of an experiment?
Answers
After an experiment, leftover reagents and products should be handled and disposed of properly to ensure safety and environmental responsibility.
Here are guidelines on what to do with leftover reagents and products:
Leftover Reagents If the reagent is still usable and stable, you may consider storing it appropriately for future use. Make sure to label the container clearly with the reagent's identity, concentration, and date. If the reagent is no longer needed or has expired, check if it can be safely disposed of down the sink or in regular waste according to local regulations and guidelines. Some reagents may require special disposal procedures due to their hazardous nature. If the reagent is hazardous or poses a risk to human health or the environment, it should be handled as hazardous waste. Contact your institution or a local waste management facility for guidance on proper disposal methods for hazardous waste.Products of an Experiment:If the products are desired and have value, they can be collected, purified, and stored for further use or analysis. If the products are not needed or have no further use, check if they can be safely disposed of down the sink or in regular waste following local regulations. If the products are hazardous, toxic, or potentially harmful, they should be treated as hazardous waste. Contact your institution or a local waste management facility for guidance on proper disposal methods for hazardous waste.It is important to prioritize safety and environmental considerations when handling and disposing of leftover reagents and products. Follow the guidelines provided by your institution, regulatory agencies, and local waste management authorities to ensure proper handling and disposal practices.
Learn more about environmental responsibility visit:
https://brainly.com/question/11802784
#SPJ11
After an experiment, leftover reagents and products should be handled and disposed of properly to ensure safety and environmental responsibility.
Here are guidelines on what to do with leftover reagents and products:
Leftover Reagents
If the reagent is still usable and stable, you may consider storing it appropriately for future use. Make sure to label the container clearly with the reagent's identity, concentration, and date.
If the reagent is no longer needed or has expired, check if it can be safely disposed of down the sink or in regular waste according to local regulations and guidelines. Some reagents may require special disposal procedures due to their hazardous nature.
If the reagent is hazardous or poses a risk to human health or the environment, it should be handled as hazardous waste. Contact your institution or a local waste management facility for guidance on proper disposal methods for hazardous waste.
Products of an Experiment:
If the products are desired and have value, they can be collected, purified, and stored for further use or analysis.
If the products are not needed or have no further use, check if they can be safely disposed of down the sink or in regular waste following local regulations.
If the products are hazardous, toxic, or potentially harmful, they should be treated as hazardous waste. Contact your institution or a local waste management facility for guidance on proper disposal methods for hazardous waste.
It is important to prioritize safety and environmental considerations when handling and disposing of leftover reagents and products. Follow the guidelines provided by your institution, regulatory agencies, and local waste management authorities to ensure proper handling and disposal practices.
Learn more about environment:
brainly.com/question/11802784
#SPJ11
587. mL of 0.00531 M NaI (aq) is combined with 840. mL of 0.00536 M Pb(NO3)2 (aq). Determine if a precipitate will form given that the Ksp of Pbl2 is 1.40x10-8.
a. Precipitation will not occur because Qsp > Ksp
b. Precipitation will occur because Qsp > Ksp
c. Precipitation will occur because Qsp = Ksp
d. Precipitation will not occur because Qsp < Ksp
e. Precipitation will occur because Qsp < Ksp
Answers
The formation of a precipitate is possible when the product of the ionic concentrations exceeds the Ksp value. Qis is the reaction quotient, which is the ionic product (IP) in a solution.
To determine whether a precipitate will occur, the reaction quotient (Qis) must be compared to the solubility product constant (Kip). The correct option is (d) Precipitation will not occur because Qis < Kip. The calculations are provided solution below; Qis = [Pb2+] [I–]2Moles of NaI = 0.587 L × 0.00531 mol/L = 0.00313 mol Moles of Pb(NO3)2 = 0.840 L × 0.00536 mol/L = 0.00451 mol[Pb2+] = 0.00451 mol / (0.587 L + 0.840 L) = 0.00327 M[I–] = 0.00313 mol / (0.587 L + 0.840 L) = 0.00226 MQsp = (0.00327 M) × (0.00226 M)2 = 1.72 × 10–8 Kip = 1.4 × 10–8As Qsp is less than Ksp, a precipitate will not form. Therefore, the correct option is (d) Precipitation will not occur because Qis < Ksp.
learn more about solution here.
https://brainly.com/question/1616939
#SPJ11
I need help with Molar Ratio
______SO2(g) +_____O2(g) ------> _____SO3(g)
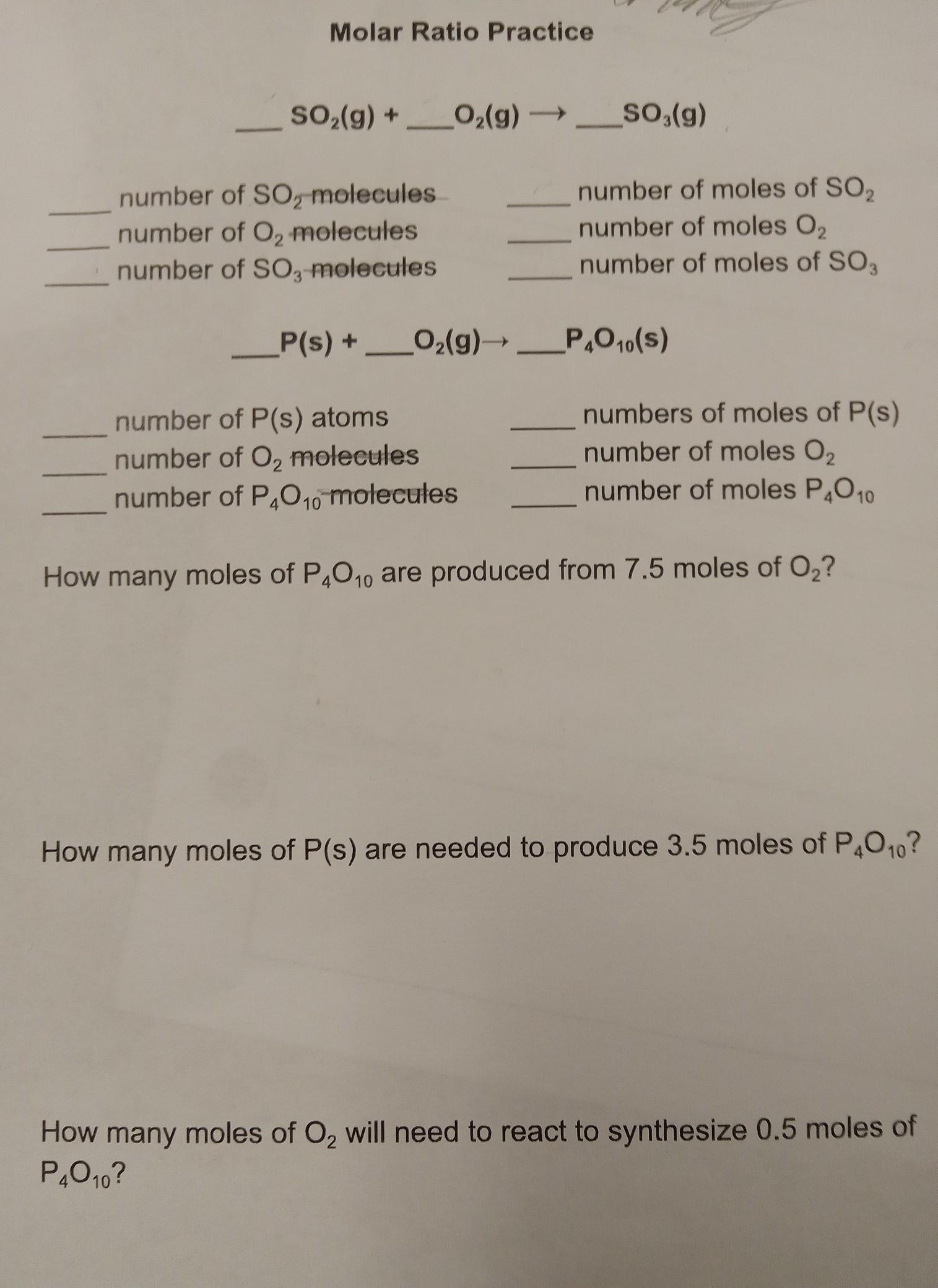
Answers
2 SO₂ (g) + O₂ (g) ---> 2 SO₃ (g)
1.204 * 10²⁴ number of SO₂ molecules = 2 number of moles of SO₂
6.02 * 10²³ number of O₂ molecules = 1 number of moles O₂
1.204 * 10²⁴ number of SO₃ molecules = 2 number of moles of SO,
4 P(s) + 5 O₂ (g) ----> P₄O₁₀ (S)
2.408 * 10²⁴ number of P(s) atoms = 4 numbers of moles of P(s)
3.01 * 10²⁴ number of O₂ molecules = 5 number of moles O₂
6.02 * 10²³ number of moles P₄O₁₀ = number of P₄O₁₀ molecules
1.5 moles of P₄O₁₀ are produced from 7.5 moles of O₂.
14 moles of P(s) are needed to produce 3.5 moles of P₄O₁₀.
2.5 moles of O₂ will need to react to synthesize 0.5 moles of P₄O₁₀.
What is the mole ratio of the given reactions?The mole ratio of the given reactions is obtained from their equations of reaction.
1. 2 SO₂ (g) + O₂ (g) ---> 2 SO₃ (g)
The mole ratio is 2 : 1 : 2
1 mole of atoms or molecules contains 6.02 * 10²³ particles.
Hence, the number of particles is obtained by multiplying the number of moles by 6.02 * 10²³.
4 P(s) + 5 O₂ (g) ----> P₄O₁₀ (S)
7.5 moles of O₂ will produce 7.5/5 moles of P₄O₁₀ = 1.5 moles of P₄O₁₀
3.5 moles of P₄O₁₀ will be produced by 3.5 * 4 moles of + = 14 moles of P(s)
0.5 moles of P₄O₁₀ will be produced by 0.5 * 5 moles of O₂ = 2.5 moles of O₂
Learn more about mole ratio at: https://brainly.com/question/30632038
#SPJ1
How does density affect sinking and floating in water?
Answers
Answer:
Density is a measure of how heavy something is compared to its size.
If an object is more dense than water it will sink when placed in water, and if it is less dense than water it will float.
Density is a characteristic property of a substance and doesn’t depend on the amount of substance.
Explanation:
If you compared the weight of wood and an equal amount, or volume, of water the sample of wood would weigh less than the sample of water. This means that wood is less dense than water. Since wood is less dense than water, wood floats in water, no matter how big or small the piece of wood is.
A student might want to know why a boat made out of steel can float when steel is more dense than water. This is not an easy question and requires a different approach than what students have seen so far. We do not necessarily recommend the following explanation for 5th graders but here is the idea:
An object floats when it displaces a volume of water that has a mass equal to the mass of the object. So if a material like steel is shaped into a boat and made larger and larger, it will displace more and more water. When it is large enough to displace a volume of water that has a mass equal to the mass of the boat, the boat will float.
1. On a train ride cross-country, the trees, buildings, and people appear to be speeding by the window. This is because the train is our _____
2. Scientists use the ______
system for making measurements.
3. Physical science studies the relationships between ____,____,
and ______.
4. If 6.6 tyrannosaurs weigh 30,000 kg, then what does one tyrannosaur weigh?
5. For most activities, the ___
makes a handy frame of reference.
Answers
On a train ride cross-country, the trees, buildings, and people appear to be speeding by the window. This is because the train is moving in the forward direction.
Scientists use the shared system for making measurements. This system is also called system of international.
Physical science studies the relationships between natural but non-living objects. Physical science is ordinarily thought of as consisting of four broad areas: astronomy, physics, chemistry, and the Earth sciences.
If 6.6 tyrannosaurs weigh 30,000 kg, then one tyrannosaur weigh about 4545 kg. For most activities, the earth makes a handy frame of reference.
Learn more about science here: https://brainly.com/question/17216882
#SPJ1
the amount of thermal energy requires to raise temperature of one kilogram of a substance by 1 C is known as
Answers
Determine the entropy change when 2.40 mol hi(g) condenses at atmospheric pressure.
Answers
The entropy change for 2.40 moles of HI when it condenses at atmospheric pressure is -199.968 J/mol K
What is the entropy change?Entropy change is defined as the measure of change of disorder or randomness in a thermodynamic system.
Mathematically, it is given as
\(\Delta S = \frac{\Delta H}{T}\)
where ∆S = entropy change
∆H = change in enthalpy
T = temperature
For HI,
Enthalpy of vaporization of HI, \(\Delta H_{vap}\) = 19.8 kJ/mol
We also know
Enthalpy of vaporization of HI, \(\Delta H_{vap}\) = - Enthalpy of condensation of HI, \(\Delta H_{cond}\)
\(\Delta H_{cond}\) = -19.8 kJ/mol
HI condenses at -35.36°C
Temperature, T = -35.36°C + 273 = 237.64 K
Substitute in the entropy change formula,
\(\Delta S = \frac{-19.8}{237.64}\)
\(\Delta S\) = -0.08332 kJ/mol K = -83.32 J/mol K
1 mole of HI has entropy change = -83.32 J/mol K
2.4 moles of HI have entropy change = 2.4 × (-83.32) = -199.968 J/mol K
Thus, The entropy change for 2.40 moles of HI when it condenses at atmospheric pressure is -199.968 J/mol K
Learn more about entropy change:
https://brainly.com/question/6364271
#SPJ4
Which gas law relates pressure and volume?
- Is there a direct or indirect relationship?
- What variable is held constant?
Answers
Gas laws are the relation between pressure, volume, and temperature of the gas. The relation between the pressure and the volume of the gas is given by Boyle's law.
What is Boyle's law?Boyle's law gives the relation between the pressure and the volume of the gas and states that the pressure is in the inverse relationship with the volume of the gas.
When the pressure of the gas is increased the volume of the gas decreases, similarly the decreased pressure of gas results in an increased volume of gas.
Boyle's law is given as,
\(\rm V = \rm constant (\dfrac{1}{P})\)
and,
\(\rm P_{1}V_{1} = \rm P_{2}V_{2}\)
Boyle's law is only true if the moles of the gas (n) and the temperature (T) of the gaseous system are constant.
Therefore, pressure and volume are in indirect relation and the moles and the temperature are constant in Boyle's law.
Learn more about Boyle's law here:
https://brainly.com/question/1437490
#SPJ1
The positvie part of the atom found in the center of the atom