what is the systematic iupac name of the compound below? multiple choice 6-bromo-4-ethylbenzenecarboxylic acid 2-bromo-4-ethylbenzenecarboxylic acid ortho-bromo-para-ethylbenzoic acid 1-bromo-3-ethylbenzoic acid
Answers
B. 2-bromo-4-ethyl benzene carboxylic acid. The term "industrial chemical" is short form for "International Union of Pure and Applied Chemistry(iupac).
What are IUPAC guidelines?The IUPAC nomenclature is based on naming the longest continuous or ring-shaped chain of carbon atoms linked by a single bond within a given molecule. Prefixes or suffixes are used in accordance with a predetermined order to identify all deviations, including those involving multiple bonds or atoms other than carbon and hydrogen.
The IUPAC name is it alphabetical?The base name is written first, then the name of the compound is spelt out in alphabetical order (derived from the number of carbons in the parent chain). The name has no spaces in it.
Learn more about prefix here:
https://brainly.com/question/14161952
#SPJ4
Related Questions
did scientists accept wegeners theory? why or why not?
Answers
Answer: No, scientist did not accept his theory of continental drift
Explanation:His theory wasn't accepted because he thought that the force of Earth's spin was sufficient to cause the continents to move, but geologist knew that rocks are too strong for this to happen. I hope this helps!
What is required to bring about a phase change?
an increase in energy only
a decrease in energy only
an increase or decrease in pressure
an increase or decrease in energy
What changes must a GAS undergo to become a LIQUID?
CONDENSATION; increase in energy
CONDENSATION; decrease in energy
EVAPORATION; increase in energy
EVAPORATION; decrease in energy
Answers
This refers to the process where water vapor becomes liquid. It is the opposite of evaporation, where liquid water becomes a vapor. Condensation happens one of two ways: Either the air is cooled to its dew point or it becomes so saturated with water vapor that it cannot hold any more water.
This means that the gas must lose energy and the particles must come closer together to form a liquid.
Learn more about CONDENSATION on
https://brainly.com/question/29509113
#SPJ1
Answer: To - What is required to bring about a phase change?
An increase or decrease in energy
Explanation: I took the test

If an electron is confined in a 10 nm box, calculate
its energy in the ground state and 15t
excited state
If an electron is confined in a 10 nm box, calculate
its energy in the ground state and 1st
excited state
Answers
The energy in the ground state of the electron confined in a 10 nm box is approximately 10.89 eV, and the energy in the first excited state is approximately 43.56 eV.
To calculate the energy of an electron confined in a 10 nm box, we can use the formula for the energy levels of a particle in a one-dimensional infinite potential well:
E_n = (n^2 * h^2) / (8 * m * L^2)
where:
E_n is the energy of the nth energy level,
n is the quantum number of the energy level (n = 1 for the ground state),
h is the Planck's constant (6.626 x 10^-34 J·s),
m is the mass of the electron (9.10938356 x 10^-31 kg),
L is the length of the box (10 nm = 10 x 10^-9 m).
Let's calculate the energy in the ground state (n = 1) and the first excited state (n = 2):
For the ground state (n = 1):
E_1 = (1^2 * h^2) / (8 * m * L^2)
Substituting the values:
E_1 = (1^2 * (6.626 x 10^-34 J·s)^2) / (8 * (9.10938356 x 10^-31 kg) * (10 x 10^-9 m)^2)
Calculating this expression will give us the energy in the ground state.
For the first excited state (n = 2):
E_2 = (2^2 * h^2) / (8 * m * L^2)
Substituting the values:
E_2 = (2^2 * (6.626 x 10^-34 J·s)^2) / (8 * (9.10938356 x 10^-31 kg) * (10 x 10^-9 m)^2)
Calculating this expression will give us the energy in the first excited state.
Please note that the energies calculated will be in joules (J). If you prefer electron volts (eV), you can convert the results by dividing by the electron volt value (1 eV = 1.602 x 10^-19 J).
Performing the calculations:
For the ground state:
E_1 = (1^2 * (6.626 x 10^-34 J·s)^2) / (8 * (9.10938356 x 10^-31 kg) * (10 x 10^-9 m)^2) ≈ 1.747 x 10^-18 J
For the first excited state:
E_2 = (2^2 * (6.626 x 10^-34 J·s)^2) / (8 * (9.10938356 x 10^-31 kg) * (10 x 10^-9 m)^2) ≈ 6.987 x 10^-18 J
Converting the energies to electron volts (eV):
E_1 ≈ 10.89 eV (rounded to two decimal places)
E_2 ≈ 43.56 eV (rounded to two decimal places)
Therefore, the energy in the ground state of the electron confined in a 10 nm box is approximately 10.89 eV, and the energy in the first excited state is approximately 43.56 eV.
Learn more about electron from the given link!
https://brainly.com/question/13998346
#SPJ11
an arctic weather balloon is filled with 24.6l of helium gas inside a prep shed. the temperature inside the shed is 7 degrees celsius. the balloon is then taken outside, where the temperature is 7 degrees celsius. calculate the new volume of the balloon. you may assume the pressure on the balloon stays constant at exactly 1 atm. round your answer to 3 significant digits.
Answers
The balloon's new volume is 24.6L. An arctic weather balloon being inflated using 24.6 litres of helium gas in a prep shed. The shed is seven degrees Celsius inside.
When the balloon is hauled outside, it is seven degrees Celsius outside. Any three-dimensional solid's volume is equal to how much room it occupies. One of these solids can be a cube, a cuboid, a cone, a cylinder, or a sphere. Chemical compounds are composed of a large number of comparable molecules (or molecular entities), which are composed of atoms from various elements bonded together by chemical bonds. Because of this, a molecule composed of atoms from a single element is not a compound.
v1/t1 = v2/t2
24.6/7 = v2/7
v2 = 24.6L
v1 = v2
Learn more about helium gas here
https://brainly.com/question/26408362
#SPJ4
name as many tv shows as you can!!!
if u name more than 10 you get brainilest!!
Answers
Answer:
Vampire diaries Freinds Twilight My little pony Thats it
Explanation:
Answer:
spongebob square pants
family guy
american dad
the cleveland show
teen titans go
robot chicken
the loud house
the Cassandras
the big bang theory
steven universe
Explanation:
Calculate the energy change associated with the transition from n=4 to n=1 in the
hydrogen atom.
A) + 3. 55 10-18 J Absorption
B) +2. 04 x 10-18) Emmision
C)-3. 55 x 10-18) Absorption
D) -2. 04 x 10-18] Emmision
Answers
Answer: D) -2.04 x \(10^{-18}\) , Emission
Explanation:
Use Bohr's equation for energy produced from a change in orbital levels.\(-2.178*10^{-18} (\frac{1^{2}}{n_{final}^{2} } - \frac{1^{2} }{n_{initial} ^{2} } )\)\(=E\)
Substitute and you will get your answer!
Is the correct answer.

Why would you rather have hot cocoa than lemonade on a cold day? (The lesson is called heat transfer)
Answers
Answer:
you would more than likely have hot coca.
Explanation:
Because when its cold out you don't want something cold, its common sense lol.
Question 7 What is the molarity for the following solution: 5. 50 L of 13. 3-MH₂CO (the formaldehyde used to "fix" tissue samples)? (A) 0. 022 mol/L (B) 13. 3 mol/L 2. 2 mol/L D) 0. 0133 mol/L 3 Points
Answers
The molarity of a solution is calculated by dividing the number of moles of solute by the volume of the solution in litres.
Therefore, the molarity of the H₂CO solution is 13.30 mol/L.
In this case, we have 5.50 L of a 13.3 M H₂CO solution. To find the molarity, we need to calculate the number of moles of H₂CO and divide it by the volume of the solution.
The formula weight of H₂CO is 30.03 g/mol. To convert from molarity to moles, we multiply the molarity by the volume in liters:
13.3 mol/L × 5.50 L = 73.15 mol
So we have 73.15 moles of H₂CO in 5.50 L of solution.
Finally, to find the molarity, we divide the number of moles by the volume of the solution:
73.15 mol ÷ 5.50 L = 13.30 mol/L
To know more about solute refer to this:
https://brainly.com/question/8851236
#SPJ11
the processing time of a chemical relaxer is affected by
Answers
1. Hair Type and Texture: The natural texture and type of hair play a significant role in determining the processing time. Coarser and thicker hair generally requires a longer processing time compared to fine or thin hair.
2. Desired Result: The desired level of straightening or relaxation also affects the processing time. If a more significant change is desired, the relaxer may need to be left on for a longer duration.
3. Relaxer Strength: Different relaxers have varying strengths, such as mild, regular, or super. The strength of the relaxer chosen can impact the processing time. Stronger relaxers may require shorter processing times, while milder relaxers may need longer processing times.
4. Hair Condition: The overall condition and health of the hair can impact the processing time. If the hair is damaged, over-processed, or chemically treated, it may require a shorter processing time to avoid further damage.
5. Manufacturer's Instructions: It is essential to follow the instructions provided by the relaxer manufacturer. They usually provide specific guidelines regarding the processing time for optimal results and to ensure the safety of the hair and scalp.
It's crucial to note that the processing time should be determined carefully, taking into account the factors mentioned above, to achieve the desired results while minimizing the risk of hair damage. It is recommended to consult with a professional hairstylist or follow the instructions provided with the relaxer product for accurate processing time guidance.
A 5g sample of copper was heated from 10 degrees c to 50 degrees c. It absorbed 76.8 J of energy as heat. What is the specific heat of this piece of copper?
Answers
As per the given data, the specific heat of the copper sample is approximately 0.384 J/g·°C.
For the specific heat of copper, we can use the formula:
q = mcΔT
Given that:
Mass of copper (m) = 5g
Change in temperature (ΔT) = 50°C - 10°C = 40°C
Heat absorbed (q) = 76.8 J
76.8 J = 5g × c × 40°C
76.8 J = 200g°C × c
c = 76.8 J / 200g°C
c ≈ 0.384 J/g·°C
Thus, the specific heat of the copper sample is approximately 0.384 J/g·°C.
For more details regarding specific heat, visit:
https://brainly.com/question/31608647
#SPJ1
a particular liquor is 49.0% ethanol (ch3ch2oh) by volume. calculate the concentration of ethanol in molality. the density of ethanol is 0.789 g/ml; the density of water is 0.998 g/ml; assume the volumes are additive.
Answers
The molality of ethanol in the solution is 0.0165 mol/kg.
The volume of a liquor that has 49.0% ethanol by volume is unknown. Therefore, we assume that 1 L of this liquor is present. This would mean that 0.49 L of ethanol is present in 1 L of liquor.
Ethanol is known to have a density of 0.789 g/mL.
This would mean that the mass of ethanol present in 1 L of liquor would be (0.49 L) × (0.789 g/mL) = 0.387 g.
The molar mass of ethanol (C2H5OH) is 46.07 g/mol.
Therefore, the number of moles of ethanol present in 0.387 g of ethanol can be calculated as
no of moles = mass/ molar mass = (0.387 g)/(46.07 g/mol) = 0.0084 mol ethanol.
This can be used to calculate the molality of the solution, which is given as the number of moles of solute per kilogram of solvent (water).
Here, the solvent (water) volume is (1 L – 0.49 L) = 0.51 L.
The density of water is 0.998 g/mL.
This would mean that the mass of water present in 0.51 L of water would be (0.51 L) × (0.998 g/mL) = 0.509 g.
Therefore, the molality of the solution is given as :
molality = no of moles of solute/ weight of solvent
= (0.0084 mol)/(0.509 kg) = 0.0165 mol/kg.
So, the molality of ethanol in the solution is 0.0165 mol/kg.
To learn more about molality :
https://brainly.com/question/13200956
#SPJ11
A _____ ionic compound is a polyatomic ionic compound composed of three or more different elements.
Answers
Answer:
ternary
Explanation:
A ternary ionic compound is a polyatomic ionic compound composed of three or more different elements.
What is ionic compound?An ionic compound is a chemical complex made up of ions that are held together through electrostatic forces. The molecule is essentially neutral, however, it contains positively charged cations as well as negatively charged anions.
What is ternary ionic compound?An ionic compound with three components is known as a ternary ionic compound. One type of cation including one type of anion are still present in a typical ternary ionic combination. Polyatomic ions are cations, anion, or both.
Therefore, a ternary ionic compound is a polyatomic ionic compound composed of three or more different elements.
To know more about ionic compound.
https://brainly.com/question/9167977.
#SPJ2
I need help in this question pleaaaaase!
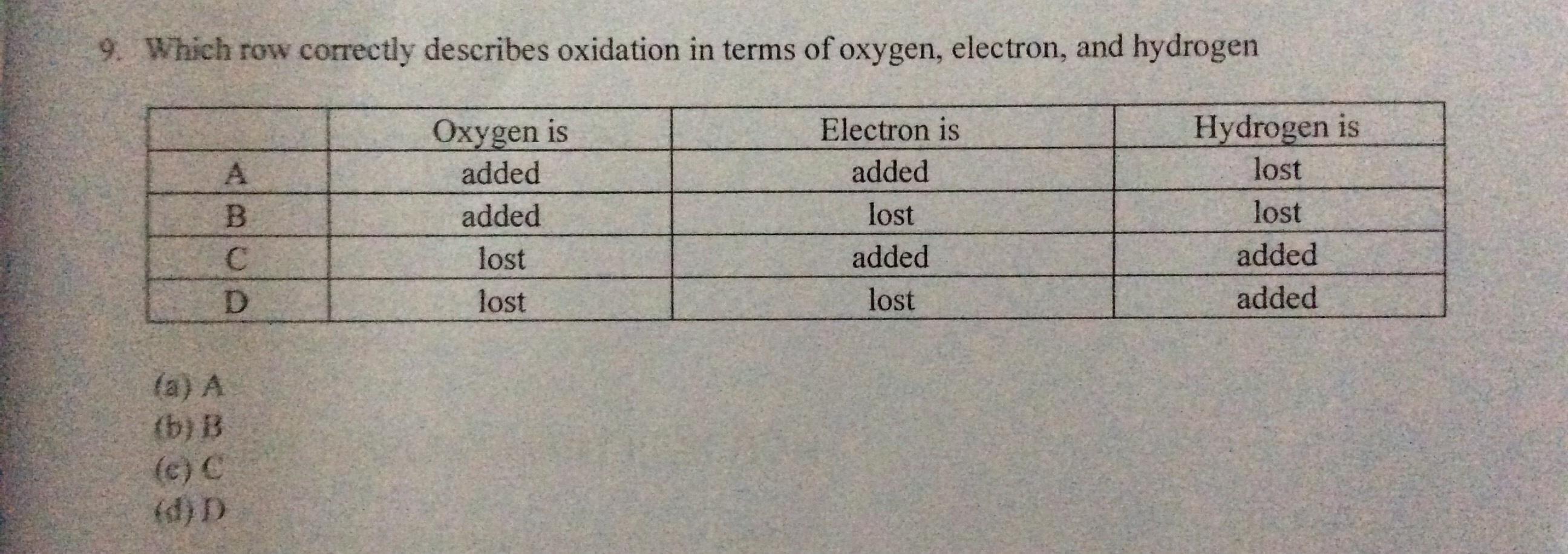
Answers
The correct definition of oxidation in terms of oxygen, electron and hydrogen is added, lost , lost, (Row B).
What is an oxidation reaction?An oxidation reaction is a reaction in which the oxidation number if the reacting species increases in a positive direction.
Oxidation can be defined in terms of oxygen as the addition of oxygen. Oxidation can be defined in terms of electron as removal of electron Oxidation can be defined in terms of hydrogen as removal of hydrogen.Therefore, the correct definition of oxidation in terms of oxygen, electron and hydrogen is added, lost , lost, (Row B).
Learn more about oxidation at: https://brainly.com/question/4222605
why isn't dna present in red blood cells
Answers
Answer:
vegetarian
Explanation:
okay so dna is actually translated to veggies and being red blood cells are red witch translates to meat and being dna is veggies it does not like meat so it does not show up in the cells being the dna is vegetarian. xoxo hope that helped, lots of love!
2. describe what happened to the ph and the carbon dioxide level with hyperventilation. how well did the results compare with your prediction?
Answers
During hyperventilation, the pH of the blood increases (becomes more alkaline), while the carbon dioxide (CO2) level decreases. These changes are consistent with the prediction.
Hyperventilation refers to an increased rate and depth of breathing, leading to the removal of excess carbon dioxide from the body. As a result, the concentration of carbon dioxide in the blood decreases. Carbon dioxide reacts with water to form carbonic acid (H2CO3), which dissociates into bicarbonate ions (HCO3-) and hydrogen ions (H+). By reducing the carbon dioxide level, there is less production of H+ ions, resulting in an increase in blood pH, making it more alkaline.
The observed changes in pH and carbon dioxide levels during hyperventilation are consistent with the predicted response. Increased ventilation causes more carbon dioxide to be expelled from the body, shifting the equilibrium of the carbonic acid-bicarbonate buffer system. As a consequence, the pH of the blood rises, leading to alkalosis. These changes can be confirmed through blood gas analysis or other diagnostic tests.
learn more about carbon dioxide here:
https://brainly.com/question/3049557
#SPJ11
Does a reaction occur when aqueous solutions of sodium sulfide and manganese(II) chloride are combined?
Oyes Ono
If a reaction does occur, write the net ionic equation.
Answers
Answer:
Yes, a reaction occurs
Explanation:
net ionic equation
Mn 2+ (aq) + S 2- (aq) --> MnS (s)
Which best explains the statement "It has no definite volume”?
Its atoms can widely spread out.
Its atoms stay closely packed.
It’s highly viscous.
It’s highly crystalline.
Answers
Answer:
D
Explanation:
The photoelectric work function of potassium is 2.3 eV . Light having a wavelength of 210 nm falls on potassium
a) Find the stopping potential for light of this wavelength. Use 6.63×10−34 J⋅s for Planck's constant, 1.60×10−19 C for the charge on an electron, and 3.00×108 m/s for the speed of light in a vacuum. Express your answer using two significant figures.
b) Find the kinetic energy of the most energetic electrons ejected
c) Find the speeds of these electrons
Answers
a) To find the stopping potential, we can use the formula:
K_max = eV_s
where K_max is the maximum kinetic energy of the ejected electrons, e is the charge on an electron, and V_s is the stopping potential. We can use the fact that the energy of a photon of light is given by:
E = hc/λ
where h is Planck's constant, c is the speed of light in a vacuum, and λ is the wavelength of the light. The work function, W, is the minimum energy required to eject an electron, and is related to the threshold frequency, f_0, by:
W = hf_0 = hc/λ_0
where λ_0 is the threshold wavelength.
For potassium, the work function is given as 2.3 eV. We can convert this to joules using:
1 eV = 1.60×10^-19 J
so W = 2.3 eV x 1.60×10^-19 J/eV = 3.68×10^-19 J.
The threshold wavelength, λ_0, can be found by rearranging the formula for the energy of a photon:
λ_0 = hc/W = (6.63×10^-34 J⋅s x 3.00×10^8 m/s)/(3.68×10^-19 J) = 5.39×10^-7 m
The threshold frequency, f_0, can be found using the formula:
f_0 = c/λ_0 = 3.00×10^8 m/s / 5.39×10^-7 m = 5.57×10^14 Hz
Now we can find the energy of a photon with wavelength λ = 210 nm = 210×10^-9 m:
E = hc/λ = (6.63×10^-34 J⋅s x 3.00×10^8 m/s)/(210×10^-9 m) = 2.99 eV
To find the stopping potential, we subtract the work function from the energy of the photon:
V_s = (E - W)/e = (2.99 eV - 3.68×10^-19 J)/(1.60×10^-19 C) = -0.425 V
Rounding to two significant figures, we get:
Stopping potential = -0.43 V
b) The kinetic energy of the most energetic electrons ejected is given by:
K_max = E - W = 2.99 eV - 2.3 eV = 0.69 eV
Converting to joules, we get:
K_max = 0.69 eV x 1.60×10^-19 J/eV = 1.10×10^-19 J
c) The speed of the electrons can be found using the formula:
K_max = 1/2 mv^2
where m is the mass of an electron and v is its speed. Solving for v, we get:
v = √(2K_max/m)
The mass of an electron is 9.11×10^-31 kg, so:
v = √(2(1.10×10^-19 J)/(9.11×10^-31 kg)) = 6.61×10^5 m/s
Rounding to two significant figures, we get:
Speed of electrons = 6.6×10^5 m/s
To know more about potential refer here https://brainly.com/question/28300184#
#SPJ11
Question 2 of 10
Which of the following best describes technology?
A. The method of thinking that scientists use.
B. The application of engineering to create useful products.
C. Something created for only scientists to use.
D. A scientific idea.
Answers
find the molecules in 75.0 liters of F2 at STP
Answers
Answer:
20.16 × 10^(23) molecules
Explanation:
We know that At STP, 22.4 L of any substance = 6.02 × 10^(23) molecules
Thus;
75 L of F2 at STP will give;
(75 × 6.02 × 10^(23))/22.4 = 20.16 × 10^(23) molecules
Number of molecules in 75.0 liters of F2 at STP = 20.16 × 10^(23) molecules
Three samples of sodium chloride are analyzed and found to contain differing percent by mass of chlorine. what does this information alone, tell us about the three samples?
Answers
Three samples of sodium chloride are analyzed and found to contain differing percent by mass of chlorine this indicates that only one solution has actual content of chlorine.
There may be differential volume of chlorine in sodium chloride solution in Nacl the mass percent of chlorine should be 60.8%.
With respect to 1 mole sodium chloride,
Mass of sodium in sodium chloride =23 g
Mass of chloride in sodium chloride =35.5 g
Total mass of sodium chloride =58.5 g
% of chlorine in NaCl =
58.5/35.5×100 = 60.85
To learn more about sodium chloride:
https://brainly.com/question/9811771
#SPJ4
when a suitable alkyl halide is treated with a nucleophile, a substitution reaction can occur in which the leaving group is replaced by a(n)
Answers
When a suitable alkyl halide is treated with a nucleophile, a substitution reaction can occur in which the leaving group is replaced by a(n) elimination reaction.
Alkyl halides and alcohols effortlessly go through nucleophilic substitution both thru SN1 or SN2 mechanism. The relative case of those tactics relies upon upon the character of the substrate (alkyl organization) in addition to leaving organization, nature of nucleophile and additionally upon the character of the solvent.
Nucleophilic Substitution response due to the fact the electrophilic alkyl halide paperwork a brand new bond with the nucleophile which substitutes for (replaces) the halogen on the alpha-carbon. Because carbon can best shape 4 bonds, the halogen ought to depart and is known as the "Leaving Group".
Read more about halogen :
https://brainly.com/question/364367
#SPJ4
Why the following happens: A bimetallic rod when heated, undergoes the change of shape. A. Because the two metals that are part of the rod have equal coefficients of linear expansion. B.Because the two metals that are part of the rod have different coefficients of linear expansion, and the one with the smallest curve has the highest value C. Because the two metals that are part of the rod have different coefficients of linear expansion, and curve to the least value D. Because the two metals that are part of the rod have similar coefficients of linear expansion.
Answers
A bimetallic rod curves in a way such that the metal with the higher coefficient of linear expansion is on the outer side (convex).
The answer is Option (C).
When a bimetallic rod is heated, it starts expanding as the molecules in the rod start vibrating more faster due to the gain in energy. This ultimately causes an increase in the average distance between the molecules, ultimately resulting in linear expansion.
The expansion ability of rods can be compared using the coefficient of Linear Expansion (α). A higher value of α between two materials denotes that it expands faster with every degree of increase in temperature.
In the case of a bimetallic strip, the two different metals used have unique values of α. So the metal with the higher α expands faster, thus resulting in the rod bending inwards with the other metal. Since they occupy the same area initially, the rod automatically starts bending to compensate for the expansion.
This property of metals is used as bimetallic strips in temperature-controlled switches, or in thermostats.
For more on Linear Expansion,
brainly.com/question/14780533
#SPJ4
what mass (in grams) of sodium bicarbonate is needed to make a 2.2 liters of a 0.45 m solution?
Answers
To find the mass of sodium bicarbonate needed to make a 2.2 liters of a 0.45 m solution, we can use the formula:
mass = volume × molarity × molar mass
where volume is in liters, molarity is in moles per liter, and molar mass is in grams per mole.
The molar mass of sodium bicarbonate is 84.01 g/mol. So, we can plug in the values into the formula:
mass = 2.2 L × 0.45 mol/L × 84.01 g/mol
mass = 83.32 g
Therefore, 83.32 grams of sodium bicarbonate is needed to make a 2.2 liters of a 0.45 m solution.
You can read more about sodium bicarbonate at https://brainly.com/question/20670487
#SPJ11
A sample of 7.75 g of Mg1OH22 is added to 25.0 mL of 0.200 M HNO3. (a) Write the chemical equation for the reaction that occurs. (b) Which is the limiting reactant in the reaction
Answers
(a) The chemical equation for the reaction that occurs between Mg(OH)\(_{2}\) and HNO\(_{3}\) can be written as: Mg(OH)\(_{2}\) + 2HNO\(_{3}\) → Mg(NO\(_{3}\))\(_{2}\) + 2H\(_{2}\)O
(b) Mg(OH)\(_{2}\) will be in excess.
To determine the limiting reactant, we need to compare the number of moles of each reactant and see which one is present in a lower amount.
First, we calculate the number of moles of Mg(OH)\(_{2}\) using its molar mass. The molar mass of Mg(OH)\(_{2}\) is 58.33 g/mol.
Number of moles of Mg(OH)\(_{2}\) = Mass of Mg(OH)\(_{2}\) / Molar mass of Mg(OH)2
= 7.75 g / 58.33 g/mol
≈ 0.133 mol
Next, we calculate the number of moles of HNO\(_{3}\) using its molarity and volume.
Number of moles of HNO\(_{3}\) = Molarity of HNO\(_{3}\) x Volume of HNO\(_{3}\)
= 0.200 mol/L x 0.0250 L
= 0.005 mol
Since the number of moles of Mg(OH)\(_{2}\) is higher than that of HNO\(_{3}\), HNO\(_{3}\) is the limiting reactant in the reaction. This means that all the HNO\(_{3}\) will be consumed, and Mg(OH)\(_{2}\) will be in excess.
You can learn more about limiting reactant at
https://brainly.com/question/26905271
#SPJ11
Perform the following operationand express the answer inscientific notation.7.15x103 x 6.10x10-5[ ? ]x10! ?)Coefficient (green)Exponent (yellow)-1Enter
![Perform the following operationand express the answer inscientific notation.7.15x103 x 6.10x10-5[ ? ]x10!](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/QLVl4FLJ0y9LtH5YrZXl2TLThmDLsNcA.jpeg)
Answers
Answer:
Explanations:
Given the operation below:
\((7.15\times10^3)\times(6.10\times10^{-5})\)Grouping the operation into standard and exponential values will give:
\(\begin{gathered} (7.15\times6.10)\times(10^3\times`0^{-5}) \\ \end{gathered}\)Simplify the result
\(\begin{gathered} (43.615)\times(10^{3+(-5)}) \\ (43.615)\times10^{-2} \end{gathered}\)Write the resulting product in standard form:
\(\begin{gathered} =4.3615\times10^1\times10^{-2} \\ =4.3615\times10^{1-2} \\ =4.3615\times10^{-1} \end{gathered}\)Hence the coefficient (green) is 4.3615 and the exponent (yellow) is -1.
What are pros and cons of hydrogen fuel cells?
Answers
Answer:
Pros: No vehicle emissions other than water vapor. Fuel economy equivalent to about twice that of gasoline vehicles. Hydrogen is abundant, and can be made from renewable energy. Cons: This space-age technology is expensive.
Answer:
The pros of hydrogen fuel cells are that they are very efficient, producing very little waste. They are also very stable, which makes them safe to use.
The cons of hydrogen fuel cells are that they can be expensive to produce and maintain, and they require a lot of energy to power them, meaning that they often aren't as efficient as they could be.
Despite these pros and cons, hydrogen fuel cells are still a very promising technology. With more research, attention, and development, they could be a powerful force in the fight against carbon emissions and global warming.
why is temperature not a chemical change
Answers
Answer:
Temperature is not a chemical change because when a substance changes in temperature, its chemical makeup is not changing.
Calculate the new volume of 1.23 mL of a gas at 32 C is subjected to drop in temperature of 20 degrees Celsius
Answers
Answer:
1,15mL = V₂
Explanation:
Based on Charle's law the volume is directely proportional to the absolute temperature in a gas under constant pressure. The equation is:
V₁T₂ = V₂T₁
Where V is volume and T absolute temperature of a gas where 1 is initial state and 2, final state.
The V₁ is 1.23mL
T₁ = 32°C + 273.15 = 305.15K
T₂ = T₁ - 20°C = 285.15K
Replacing:
1.23mL*285.15K = V₂*305.15K
1,15mL = V₂
How might synthetic products be helpful to the environment?
Answers
Answer:
synthetic products can be helpful to the environment in many ways like:
Explanation:
can help in conserving resources
can reduce pollution
can help in producing environment friendly resources
can recycle products