what is the simplest formula of a solid containing a, b, and c atoms in a cubic lattice in which a atoms occupy two corners and one body-center position, the b atoms occupy two corners, and the c atoms occupy four corners and two faces of the unit cell?
Answers
A solid with atoms a, b, and c arranged in a cubic lattice has the simplest formula possible: abc3.
Given that the unit cell's an atoms occupy two of its corners and one of its body centres, the b atoms occupy two corners, and the c atoms occupy four of its corners and two of its faces
There are eight corners, and each one has an atom in it.
Atomic number of a = 8 * 1/8 = 1
The centre b atom is the lone atom. So b = 1
Each of the six faces holds a c atom.
6 x 1/2 = 3 c atoms are present.
So when we combine them, we get the solid as abc3 formula.
To learn more about lattice click here https://brainly.com/question/29774529
#SPJ4
Related Questions
If the same large amount of heat is added to a 250 g piece of aluminum and a 150 g piece of aluminum, what will happen?
Answers
please vote me brainliest i really need it for i can do my work
Q.) Oxidation number of P in PO4³, of S in SO4² and that of Cr in Cr₂O7² are respectively:
(a) +3, +6 and +5
(b) +5, +3 and +6
(c) +3, +6 and +6
(d) +5, +6 and +6
Answers
In a compound, the sum of the oxidation numbers of all the atoms must be equal to the overall charge on the compound. In the case of PO4³-, the overall charge is -3, so the sum of the oxidation numbers of P and O must be -3. We know that the oxidation number of O is -2, so the oxidation number of P must be +3, which is option (c).
Similarly, in the case of SO4²-, the overall charge is -2, so the sum of the oxidation numbers of S and O must be -2. We know that the oxidation number of O is -2, so the oxidation number of S must be +6, which is option (c).
Finally, in the case of Cr₂O7²-, the overall charge is -2, so the sum of the oxidation numbers of Cr and O must be -2. We know that the sum of the oxidation numbers of the O atoms is -14, so the sum of the oxidation numbers of the Cr atoms must be +12. Since there are two Cr atoms, each must have an oxidation number of +6, which is option (c).
a sudden increase in end-tidal co2 may be the earliest indicator of:
Answers
A sudden increase in end-tidal CO2 (carbon dioxide) levels may be the earliest indicator of respiratory distress or failure.
End-tidal CO2 refers to the partial pressure or concentration of CO2 at the end of an exhaled breath. It is a reflection of the CO2 levels in the bloodstream. In a healthy individual, end-tidal CO2 levels are relatively stable and within a normal range. However, a sudden increase in end-tidal CO2 can indicate a problem with respiratory function. It may suggest that the body is not effectively eliminating CO2, which can occur in conditions such as hypoventilation, airway obstruction, respiratory muscle weakness, or respiratory failure.Monitoring end-tidal CO2 is commonly done in medical settings, especially during anesthesia or critical care, as it provides valuable information about a patient's ventilation and respiratory status. Detecting an abrupt increase in end-tidal CO2 can prompt early intervention and treatment to prevent further respiratory compromise and improve patient outcomes.
To know more about concentration, click here https://brainly.com/question/3045247
#SPJ11
Which phase of matter is the rarest in the solar system?
O A. Solids
O B. Plasma
O C. Gases
O D. Liquids
Answers
Answer: the answer is LIQUIDS
Answer:
D. Liquids
Explanation:
Liquid is probably the rarest state in the Universe, with the only discovered naturally occurring liquids being the Earth's surface water and our liquid metal core.
A donut has a density of 0.75 g/cm cubed and a mass of 100.0g. What is the volume of the donut?
Answers
Answer:
133.333333333 cm^3
Explanation:
Volume = Mass/Density
what is the molarity of a solution made by dissolving 1.59 mol of lithium chloride in enough water to make 2.37 l of solution
Answers
The molarity of the lithium chloride solution is 0.671 M.
Molarity is a unit of concentration used to measure the amount of solute dissolved in a given volume of solution. It is defined as the number of moles of solute dissolved in one liter of solution, and its unit is moles per liter (mol/L).
To calculate the molarity of the solution, we need to divide the number of moles of solute by the volume of solution in liters.
Molarity = moles of solute/volume of solution in liters
In this case, we are given that 1.59 mol of lithium chloride is dissolved in enough water to make 2.37 L of solution. Therefore, the molarity will be calculated as
Molarity = 1.59 mol / 2.37 L
Molarity = 0.671 M
To know more about molarity here
https://brainly.com/question/8732513
#SPJ4
What compound is formed from Ca2+ and Cl1-
Answers
Answer:
You get CaCl2 (Calcium chloride)
Explanation:
Calcium has a +2 charge and chlorine has a -1 charge. You need the net ionic charge to equal zero so you need two chlorine atoms to have the +2 cancel out. You end up with CaCl2.
What is water's density at 91 ∘c? assume a constant coefficient of volume expansion.
Answers
Answer:
982.5 kg/m³
Explanation:
When the temperature of a fluid increases, it dilates, and because of the variation of the volume, it's density will vary too. The density can be calculated by the expression:
ρ₁ = ρ₀/(1 + β*(t₁ - t₀))
Where ρ₁ is the final density, ρ₀ the initial density, β is the constant coefficient of volume expansion, t₁ the final temperature, and t₀ the initial temperature.
At t₀ = 4°C, the water desity is ρ₀ = 1,000 kg/m³. The value of the constant for water is β = 0.0002 m³/m³ °C, so, for t₁ = 93°C
ρ₁ = 1,000/(1 + 0.0002*(93 - 4))
ρ₁ = 1,000/(1+ 0.0178)
ρ₁ = 982.5 kg/m³
In a chemical equation what does the downward pointing arrow signify ?
Answers
Answer:
spontaneously precipitateAn arrow pointing down (as in PbI2?) indicates that the product will spontaneously precipitate from the solution. ("Precipitate," in this sense, means the formation of a solid from the combination of two aqueous solutions.)
Draw the skeletal structure of the given sugar with the carbon chain having the zigzag staggered conformation and using the wedge/dashed tool to establish the configurations of the chiral carbons. Make sure the tapered end of the wedge/dashed bond is bonded to the chiral carbon. Hydrogens bonded to carbon are understood and should not be shown. In other words, you should NOT use the X-tool. One last warning - each chiral carbon should have 2 stick bonds and one wedge or dashed bond.
1. D Ribose
1b. Substitutive name
2. D mannose
2b. Substitutive name
Answers
1. Here is the skeletal structure of D Ribose with the zigzag staggered conformation and using the wedge/dashed tool to establish the configurations of the chiral carbons:
O
|
C--H
\
C--H
/ \
C C--O--H
\ /
C--H
/
O--H
The substitutive name for D Ribose is D-ribofuranose.
2. Here is the skeletal structure of D Mannose with the zigzag staggered conformation and using the wedge/dashed tool to establish the configurations of the chiral carbons:
O
|
C--H
\
C--H
/ \
C C--O--H
\ /
C--OH
/
O--H
The substitutive name for D Mannose is D-mannopyranose.
For drawing the skeletal structures, I recommend using chemistry drawing software like ChemDraw, or drawing them manually following the conformation and stereochemistry rules described in your question.
Learn more about skeletal here:
https://brainly.com/question/29165067
#SPJ11
cwhat is the difference between chemical properties and physical properties?
Answers
Why are we able to see planets at night?
Answers
Answer: Because planets do not have nuclear fusion, they do not produce their own light. Instead, they shine with light reflected from a star. When we see planets in the night sky, such as Venus, the so-called "Evening Star," we're seeing reflected sunlight.
Explanation:
PLEASE HELP ASAP!!!!
ANSWERS FOR THE LAST PHOTO
W
X
Y
Z

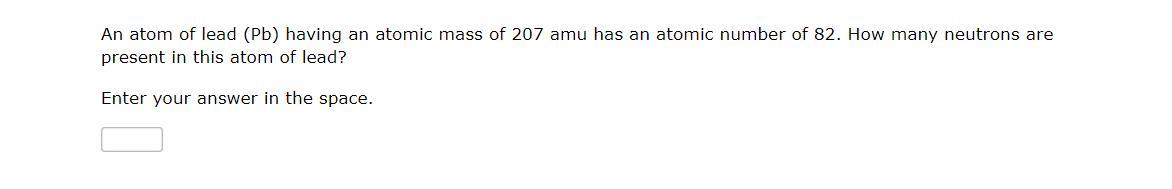



Answers
An atom is made up of three subatomic particles namely; protons, neutrons and electrons.
The proton is the positively charged particle in the nucleus of the atom while the electron is the negatively charged particle surrounding the nucleus.
The number of neutrons can be calculated by subtracting the atomic number of the element from the mass number.
In question 1 of the above image, no of neutrons = 39 - 19 = 20 neutronsIn question 2, no of neutrons = 207 - 82 = 125 neutronsLearn more about neutrons at: https://brainly.com/question/28992636
#SPJ1
2. His_________would take place tomorrow. (bury)
3.His________of words is good. (choose)
4. I received the gift with_______(please)
Answers
Answer:
1. burial
2. Choice
3. pleasure
Explanation:
Mark brainliest! Please!
. How can you identify unsaturated, saturated and supersaturated solutions?
Answers
Wavelength of yellow light with frequency of 5.2x10 14
Answers
Answer:
5.77x10^-7 m or 577 nm (nanometers)
Explanation:
The wavelength, λ, and frequency, ν, of light are described by the equation:
c = λν
where c is the speed of light.
c = 3.0x10^8 m/s
v = 5.2x10^14
λ = c/v
λ = (3.0x10^8 m/s)/(5.2x10^14) = 5.77x10^-7 m
since 1 m = 10^9nm, we can express this as 577 nm (nanometers)
577 nm. This is in the yellow light span of wavelengths.
what is the name of the triatomic form of oxygen (o3)?
Answers
The triatomic form of oxygen, with three oxygen atoms bonded together, is called ozone (O₃).
Ozone is a pale blue gas that has a distinctively sharp odor, and it is formed naturally in the atmosphere by a chemical reaction between oxygen molecules and ultraviolet light.
Ozone is also formed as a byproduct of some industrial processes and can be used for various applications, such as in water treatment and air purification systems.
However, high concentrations of ozone can be harmful to human health and the environment, and it is classified as a pollutant. Therefore, it is important to regulate and monitor ozone levels to maintain a healthy and safe environment for all living beings.
To know more about ozone visit:
https://brainly.com/question/14330630
#SPJ11
Part 1
The element with a valence electron configuration of 3s23p6 is in group and period .
(2) The element with a valence electron configuration of 2s22p4 is in group and period .
Part 2
What is the name of the element with a valence electron configuration of 4s2?
(2) What is the name of the element with a valence electron configuration of 2s22p5?
Answers
The element with this electron configuration is carbon (C) and the element with a valence electron configuration of \(2s^22p^5\) is called fluorine (F) and element with a valence electron configuration of \(4s^2\) is called calcium (Ca).
The element with a valence electron configuration of \(3s^23p^6\) is in Group 18 (also known as Group 8A or Group 0) and Period 3. This configuration indicates that the element has a completely filled outer electron shell, known as a noble gas configuration. Elements in Group 18 are called noble gases, and they are known for their stable and non-reactive nature due to their full outer electron shells. The element with this electron configuration is argon (Ar), which has 18 electrons in total.
The element with a valence electron configuration of \(2s^22p^4\) is in Group 14 (also known as Group 4A) and Period 2. This configuration indicates that the element has four valence electrons in its outermost energy level. Elements in Group 14 typically form covalent bonds and can have diverse chemical properties.
(1) The element with a valence electron configuration of \(4s^2\) is called calcium (Ca). Calcium is an alkaline earth metal and belongs to Group 2 on the periodic table. It has a total of 20 electrons.
(2) The element with a valence electron configuration of \(2s^22p^5\) is called fluorine (F). Fluorine is a halogen and belongs to Group 17 on the periodic table. It has a total of 9 electrons.
Learn more about electron configuration here:
brainly.com/question/29157546
#SPJ11
A man heats a balloon in the oven. If the balloon initially has a pressure of 860. 0 torr and
a temperature of 20. 0 °C, what will the temperature (in Kelvin) of the balloon be after he
increases the pressure to 3. 00 atm? (Hint: Convert to atmospheres). Do not include
units in your answer.
Answers
The temperature of the balloon after increasing the pressure to 3.00 atm is 608 K.
First, we need to convert the initial pressure from torr to atm, which is 860.0 torr/760 torr/atm = 1.13 atm.
Using the combined gas law, we can solve for the new temperature:
(P₁x V₁)/T₁ = (P₂x V₂)/T₂
Where P₁ = 1.13 atm, V₁ is constant, T₁ = 20.0 + 273.15 K (convert from Celsius to Kelvin), P₂ = 3.00 atm, and we want to solve for T₂.
Substituting the values and solving for T₂:
T₂ = (P₂ x V₁ x T₁)/(P₁ x V₂) = (3.00 atm x V1 x 293.15 K)/(1.13 atm x V₂)Since V₁ and V₂ are equal (since it is the same balloon), we can simplify to:
T₂ = (3.00 atm x 293.15 K)/1.13 atm = 608 KTherefore, the temperature of the balloon after increasing the pressure to 3.00 atm is 608 K.
To learn more about temperature, here
https://brainly.com/question/11464844
#SPJ4
Calculate the atomic mass of lead. The four lead isotopes have atomic masses and relative abundances of 203.973 amu (1.4%), 205.974 amu (24.1%), 206.976 amu (22.1%) and 207.977 amu (52.4%).
Answers
Work:
203.973 amu *(0.014) = 2.855 amu
205.974 amu *(0.241) = 49.639 amu
206.976 amu *(0.221) = 45.741 amu
207.977 amu *(0.524) = 108.979 amu
2.855 + 49.639 + 45.741 + 108.979 = 207.217 amu
The atomic mass of lead obtained from its four isotopes with atomic masses and relative abundance of 203.973 amu(1.4%), 205.974 amu(24.1%), 206.976 amu(22.1%), and 207.977 amu(52.4%), is 207.217 amu.
The atomic mass of lead is given by:
\( A = m_{1}\%_{1} + m_{2}\%_{2} + m_{3}\%_{3} + m_{4}\%_{4} \) (1)
Where:
m: is the atomic mass of the isotopes
%: is the abundance percent of the isotopes
After entering the given values into equation (1) we have:
\( A = 203.973 \:amu*1.4\% + 205.974 \: amu*24.1\% + 206.976\: amu*22.1\% + 207.977\: amu*52.4\% \)
To calculate the atomic mass we need to change the percent values to decimal ones
\( A = 203.973 \:amu*0.014 + 205.974 \: amu*0.241 + 206.976\: amu*0.221 + 207.977\: amu*0.524 = 207.217 amu \)
Therefore, the atomic mass of lead is 207.217 amu.
You can learn more about atomic mass here:
https://brainly.com/question/13672279?referrer=searchResultshttps://brainly.com/question/11096711?referrer=searchResultsI hope it helps you!

PLeas hurry
Which of the following matches a human action with its impact?
Construction of dam : Scarcity of water for animals
Use of fertilizers : Increase in the population of aquatic animals
Decrease in honeybee population : Increase in plant population
Construction of roads : Improvement in quality of water in local lakes
Answers
Answer:
construction aka A
Explanation:
got 100
Limiting Reagents: Practice Problem 2: Starting from Grams
I have walked you through my thought process as I worked through the problem below. Each thought I had - I wrote it as a step. You need to show work in order to receive full credit!
You will need your periodic table for this problem:
An unbalanced chemical equation is given as: ___Na + ___O2 --> ___Na2O
If you have 100 g of sodium and 60 g of oxygen…
A. Find the number of grams of sodium oxide produced by each reactant.
1st Step - Balance the Equation
2nd Step - Convert mass of Na to moles of Na
3rd Step - Convert moles of Na to moles of Na2O (hint: see ratio between Na and Na2O in your balanced equation)
4th Step - Convert moles of Na2O to grams of Na2O
5th Step - Repeat Steps 2 - 4 by substituting Na with O2
6th Step - Determine the limiting reactant and the amount of sodium oxide it produces
7th Step - The amount that it produces is the grams of sodium oxide that will be produced in the reaction.
B. Find the mass of excess reactant left over at the conclusion of the reaction.
1st Step - State what the excess reactant is from the previous problem - no quantity needed. (hint: It is not the limiting reagent)
2nd Step - Determine what is being asked in the question.
The question is asking how much of the excess reactant is needed to produce the amount of sodium oxide determined by the limiting reactant.
3rd Step - Convert grams of product to grams of excess reactant
4th Step - Determine the difference between the amount given in the initial problem and the amount used. There should be excess left over - which is why it is the excess reactant.
Answers
The number of grams of sodium oxide produced by each reactant is;
from Na = 134.8 gfrom O₂ = 232.5 gThe limiting reactant is Na and O₂ is the excess reactant.
The amount of excess reactant left at the end of the reaction is 25 g.
What are limiting reagents?Limiting reagents are reagents that are used up in a reaction.
The limiting reagent of the reagent is determined as follows:
Equation of reaction: 4 Na (s) + O₂ (g) ----> 2 Na₂O (s)
From the given data:
There are 100 g of sodium and 60 g of oxygen
Molar mass of Na = 23 g/mol
Molar mass of O₂ = 32 g/mol
molar mass of Na₂O = 62 g/mol
moles = mass / molar mass
Moles of Na = 100/23 moles
mass of sodium oxide produced from Na = 100/23 * 2/4 * 62
mass of sodium oxide produced from Na = 134.8 g
Moles of O₂ = 60/32 moles
Mass sodium oxide produced from O₂ = 60/32 * 2/1 * 62
Mass sodium oxide produced from O₂ = 232.5 g
The limiting reactant is Na and O₂ is the excess reactant.
b. Amount of excess reactant used = 100/23 * 1/4 * 32
Amount of excess reactant used = 35 g
Amount of excess reactant left = 60 - 35
Amount of excess reactant left = 25 g
Learn more about limiting reactants at: https://brainly.com/question/24945784
#SPJ1
If deficient, which of the following minerals would have the least effect on blood pressure?
potassium, sodium, calcium, magnesium
Answers
The minerals would have the least effect on blood pressure is calcium.
What is blood pressure?
Blood pressure is the measure of the force with which arteries are filled with blood. The force the heart muscle uses to pump blood against artery walls is referred to as systolic blood pressure.
What is minerals ?
A mineral is a naturally occurring inorganic element or compound with a recognized chemical composition, crystal structure, and physical characteristics. Common minerals include calcite, quartz, feldspar, mica, amphibole, and olivine.
Therefore, minerals would have the least effect on blood pressure is calcium.
Learn more about blood pressure from the given link.
https://brainly.com/question/14661197
#SPJ1
Answer:SODIUM
Explanation:
which of the following is true for the solubility of nacl(s) and ch4(g) in water?
Answers
Sodium chloride will be soluble in water because it is polar molecule.
A polar molecule was one who has a slight positive charge on one end and a slight negative charge on the other. A polar molecule would be a diatomic compound, such as HF, that contains a polar covalent link.
We refer to something as being polar when it differs at either end. Some molecules also contain positive as well as negative ends; these are known as polar molecules. If not, we refer to them as non-polar. Polar objects can both attract and repel one another.
Pyramidal and V-shaped molecules were typically considered to be polar. Linear molecules, on the other hand, are thought to be non-polar through nature. Because the oxygen and hydrogen atoms have different electronegativities, water would be considered to be a polar molecule.
To know more about polar molecule.
https://brainly.com/question/15173422
#SPJ4
Name the following compound: CH,CH,CH, OH CH3 CH; CH, CH, (Z)-4,5-dimethyl-4-heptenol O (E)-3,4-dimethyl-3-hepten-7-ol O (E)-4,5-dimethyl-4-hepten-1-ol O (2)-3,4-dimethyl-3-hepten-7-ol O (Z)-4,5-dimethyl-4-hepten-1-ol > A Moving to another question will save this
Answers
The name of the compound is (Z)-4,5-dimethyl-4-hepten-1-ol.
It contains a double bond (hence the "en" ending) between the 4th and 5th carbons from the end, and a hydroxyl group (-OH) attached to the 1st carbon.
The "dimethyl" prefix indicates that there are two methyl groups (-CH3) attached to the 4th carbon,
The "hepten" prefix indicates that there are seven carbons in the molecule with a double bond between the 4th and 5th carbons.
The "ol" ending indicates that it is an alcohol with the hydroxyl group attached to the 1st carbon.
Learn more about methyl groups here:
https://brainly.com/question/12904781
#SPJ11
determine the PH of a solution containing 0.05moldm-3 NACO3
Answers
The solution is going to have a pH of 12.7.
What is the pH?The pH uses a logarithmic scale with 7 as neutral, where lower values are more acidic and higher ones are more alkaline, to represent the acidity or alkalinity of a solution. The hydrogen ion concentration (c), expressed in moles per litre, determines the pH, which is equal to log10(c).
Now we know that;
pOH = - log(0.05M)
pOH = 1.3
pH = 14 - 1.3
= 12.7
We know that the above can be justified if we remember that pH +
pOH = 14
Learn more about pH:https://brainly.com/question/15289741
#SPJ1
How many moles of hydrochloric acid are required to produce .40 moles of hydrogen gas?
Answers
Answer:
0.80 Moles of Hydrochloric Acid would be required to produce 0.40 Moles of hydrogen gas due to the equation ratios
Explanation:
How many moles of hydrochloric acid are required to produce .40 moles of hydrogen gas?
Hydrochloric acid = HCl
Hydrogen gas = H2
H = 1+
Cl = 1-
HCL = H2 + Cl2
2HCl = H2 + Cl2
2:1:1
0.80 = 0.40 + 0.40
which of the following substances has the greatest dipole moment? check the periodic table and recall patterns of electronegativity. responses h2o uppercase h subscript 2 end subscript uppercase o hcl upper case h c lower case l hl upper case h lower case l hf
Answers
The substance with the greatest dipole moment is HCl.
Chlorine (Cl) is more electronegative than hydrogen (H), meaning that it attracts the shared electrons in the bond towards itself to a greater extent than hydrogen does. This results in a partial negative charge on the chlorine atom and a partial positive charge on the hydrogen atom, creating a dipole moment.
On the other hand, the dipole moment of H2O is also high but less than that of HCl. This is because oxygen (O) is more electronegative than hydrogen (H), but the difference in electronegativity between the two atoms is smaller than that between hydrogen and chlorine. Therefore, the dipole moment of H2O is less than that of HCl.
Finally, HF has the lowest dipole moment among the given substances because the difference in electronegativity between hydrogen and fluorine is the smallest. Although fluorine is the most electronegative element on the periodic table, the difference in electronegativity between hydrogen and fluorine is too small to create a large dipole moment in HF.
Learn more about electronegativity at:
https://brainly.com/question/1163776
#SPJ11
A sample of xenon gas at 306 K and 0. 847 atm occupies a volume of 2. 96 L. If the pressure of the gas is decreased, while at the same time it is heated to a higher temperature, the final gas volume O will be smaller than 2. 96 L. O will be larger than 2. 96 L. O could be larger or smaller than 2. 96 L depending on the final pressure and temperature
Answers
The final gas volume, O, could be either larger or smaller than 2.96 L, depending on the final pressure and temperature. While heating the gas to a higher temperature, O could be larger than 2.96 L, but it could also be smaller.
The final gas volume, O, could be either larger or smaller than 2.96 L, depending on the final pressure and temperature. To understand why, we can look at the ideal gas law equation, PV = nRT. In this equation, P represents pressure, V represents volume, n represents the number of moles of gas, R is the gas constant, and T represents temperature.
Let's analyze the situation given in the question. We have a sample of xenon gas at a temperature of 306 K and a pressure of 0.847 atm, occupying a volume of 2.96 L. Now, if the pressure of the gas is decreased, while at the same time it is heated to a higher temperature, the final gas volume, O, could be either larger or smaller than 2.96 L.
If we decrease the pressure while keeping the temperature constant, according to Boyle's law, the volume of the gas will increase. So, in this case, O would be larger than 2.96 L. However, if we simultaneously increase the temperature while decreasing the pressure, the situation becomes more complex. The combined effect of the pressure decrease and temperature increase could lead to different outcomes for the final volume.
For example, if the pressure decrease is significant and the temperature increase is relatively small, the volume may still increase, resulting in O being larger than 2.96 L. On the other hand, if the pressure decrease is small and the temperature increase is significant, the volume may actually decrease, resulting in O being smaller than 2.96 L.
To know more about Boyle's law refer to this:
https://brainly.com/question/21184611
#SPJ11
What does the pacific tsunami warning system use to detect tsunamis ?
A. Radio signals In the air
B. Ripples in the water
C. Sensors on the ocean floor
D. Behavior of marine animals
Answers
Answer:
C
Explanation:
Their are pressure sensers in the water that will detect high pressure and set of scales that are currently detecting semic waves and trigger sierns.