what is the product of this reaction mgso4(aq) bacl2(aq)
Answers
The product of this reaction is magnesium chloride (MgCl\(_2\)) and barium sulfate (BaSO\(_4\)).
To determine the product of the reaction between MgSO\(_4\)(aq) and BaCl\(_2\)(aq), you can follow these steps:
1. Write down the reactants: MgSO\(_4\)(aq) + BaCl\(_2\)(aq)
2. Determine the possible products by switching the ions: Mg\(^{2+}\) will combine with \(Cl^-\), and \(Ba^{2+}\) will combine with \(SO_4^{2-}\).
3. Write down the possible products: \(MgCl_2\) and \(BaSO_4\)
4. Check the solubility of the products. MgCl\(_2\) is soluble, while BaSO\(_4\) is insoluble in water.
5. Write the balanced equation with the correct product: MgSO\(_4\)(aq) + BaCl\(_2\)(aq) → MgCl\(_2\)(aq) + BaSO\(_4\)(s)
So, the product of the reaction between MgSO\(_4\)(aq) and BaCl\(_2\)(aq) is MgCl\(_2\)(aq) and BaSO\(_4\)(s).
Learn more about the reaction here:
https://brainly.com/question/517429
#SPJ11
Related Questions
Consider the hydrate calcium chloride dihydrate: cacl2 · 2h2o. how many hydrogen atoms are in the compound?
Answers
Consider the hydrate calcium chloride dihydrate hydrogen atoms are in the compound are four
Hydrate calcium chloride dihydrate is the excellent water soluble crystalline calcium sources used compatible with chloride and chloride compounds can conduct electricity when fused or dissolved in water and here given compound is CaCl₂ × 2H₂O
So in that compound the hydrogen atom are four because this compound or this equation is balanced so in that compound four hydrogen atom are present
Know more about hydrogen
https://brainly.com/question/13954550
#SPJ1
Answer:
4
Explanation:
In a tank, 27 L He at 25ºC and 101.3 kPa and 12 L O2 at 25ºC and 101.3 kPa are pumped into a tank with a volume of 8.0 L. Calculate the partial pressure of each gas and the total pressure in the tank at 25ºC.
Answers
Total pressure = 4.9 atm
Partial pressure of neon = 3.4 atm
Partial pressure of oxygen = 1.5 atm
What are the mole fractions?We know that the partial pressure could be obtained as the product of the mole fraction and the total pressure thus we have to obtain the total pressure by the use of the partial pressures.
For the number of moles of helium;
P = 101.3 kPa or 0.99 atm
T = 25ºC or 298 K
V = 27 L
n = PV/RT = 0.99 atm * 27 L/0.082 * 298 K = 26.73/24.44 = 1.1 moles
Number of moles of oxygen
P = 101.3 kPa or 0.99 atm
T = 25ºC or 298 K
V = 12 L
n = PV/RT = 0.99 atm * 12 L/0.082 * 298 K =11.88 /24.44 = 0.5 moles
Total number of moles = 1.1 moles + 0.5 moles = 1.6 moles
Total pressure is obtained from;
nRT/V
= 1.6 moles * 0.082 * 298/8
= 4.9 atm
Partial pressure of neon = 1.1 moles/1.6 moles * 4.9 atm = 3.4 atm
Partial pressure of oxygen = 0.5 moles/1.6 moles * 4.9 atm = 1.5 atm
Learn more about partial pressure:https://brainly.com/question/13199169
#SPJ1
Meh peepss halp will mark brainliest!!!! u-u

Answers
Answer:
The three parts of the cell theory are as follows: (1) All living things are made up of cells, (2) Cells are the smallest units (or most basic building blocks) of life, and (3) All cells come from preexisting cells through the process of cell division. ... Today, the cell theory is considered the foundation of biology.
Explanation:
hope this help.. brainliest???
Answer:
i wanna give brailiest to the person at the top!
Explanation:
what are examples of types of chemical markers associated with dna that determines when, where and by how much genes are expressed in each cell?
Answers
examples of types of chemical markers associated with dna that determines when, where and by how much genes are expressed in each cell is epigenetic.
When, where, and how much a gene is expressed in a cell depends on the histones and epigenetic elements working together.
Who or what are histone protein factors?Eukaryotic cells have proteins called histones that serve two purposes. They help keep DNA compact and control the loosening and tightening of the DNA strands that control gene expression.
How do epigenetic factors work?Epigenetic factors are substances that alter genes to control how they are expressed. Both changes are not inheritable and do not lead to mutations. These include transient changes such as DNA remodeling, methylation and alkylation.
The field of epigenetics deals with stable phenotypic changes that are not caused by changes in the DNA sequence. Epigenetics, a name derived from the Greek prefix epi-, refers to traits that are 'above' or 'additional' to the conventional genetic base.
learn more about Epigenetic visit brainly.com/question/29659855
#SPJ4
Why are elements and compounds represented differently in chemistry?
Claim:
Evidence:
Reasoning:
Answers
Answer:
Ohhhhhh
Explanation:
Ohhhhhhhhhhhhhhh
Explain whether the molecular orbitals linked to H+ are more
affected by oxygen or nitrogen when NO-ions react with H+ ions to
form chemical bonds.
(It means HON or HNO)
Answers
The molecular orbitals linked to H+ are more affected by nitrogen (N) rather than oxygen (O) when NO- ions react with H+ ions to form chemical bonds, resulting in the formation of HNO.
In the formation of chemical bonds, the reactivity and bonding characteristics are determined by the electronic configuration and orbital interactions of the atoms involved. In the case of NO- reacting with H+, we consider the electronic configurations of oxygen (O) and nitrogen (N) atoms.
Oxygen has six valence electrons and belongs to Group 16 of the periodic table. Its electronic configuration is 1s² 2s² 2p⁴. When forming bonds, oxygen typically accepts two electrons to achieve a stable octet configuration.
Nitrogen has five valence electrons and belongs to Group 15. Its electronic configuration is 1s² 2s² 2p³. Nitrogen typically needs to gain three electrons or share three pairs of electrons to achieve a stable octet configuration.
In the case of NO-, the oxygen atom carries a negative charge (O-), making it more electron-rich than nitrogen.
learn more about molecular orbitals :
https://brainly.com/question/31828377
#SPJ4
For the frequency, 4.7 x 10^12 Hz, what is the wavelength?
Answers
When 0. 1156 g of an unknown compound that contains carbon, hydrogen and nitrogen, is reacted with oxygen, 0. 1638 g of co2 and 0. 1676 g of h2o are collected. Determine the empirical formula of this compound
Answers
The empirical formula of this unknown compound with carbon, hydrogen and nitrogen is C2HN3.
Originally, chemical formulas were generated by determining the masses of all the components that combine to create a molecule, and this resulted in two main types of chemistry formulae: molecular formulas and empirical formulas.
A compound's empirical formula offers the simplest ratio of the number of various atoms present, but the molecular formula specifies the actual number of each single element present in a molecule. It is an empirical formula if the formula is simplified. The molecular formula, which is a multiple of the empirical formula, is often employed.
Mass of compound is 0.1156 g
mass of CO2 = 0.1638 g
mass of H2O = 0.1676 g
Molecular weight of CO2 = 44 g
Molecular weight of H2O = 18 g
moles of CO2 = 0.00372
0.00372 x 12 = 0.04464 = C
H = 0.00931 x 2 = 0.01862
N = 0.1156 - (0.04464 + 0.01862) = 0.05234
Empirical formula is,
C:H:N = 0.04464 : 0.01862 : 0.05234 = 0.04 : 0.02 : 0.06
C : H : N = 2 : 1 : 3
So Empirical formula is C2HN3.
Learn more about Empirical formula :
https://brainly.com/question/1603500
#SPJ4
light behaves as a longitudinal wave
Answers
Answer:
No, light behaves as a Transverse Wave. All electromagnetic waves behave as transverse waves.
show all work please

Answers
10. The new pressure will be 1703 mmHg
11. The new volume will be 25 mL
12. The new volume will be 6767.3 mL
13i. The pressure (in mmHg) is 6826.38 mmHg
13ii. The pressure (in torr) is 6826.38 torr
13iii. The pressure (in atm) is 8.98 atm
10. How do i determine the new pressure?The new pressure can be obtain as follow:
Initial volume (V₁) = 325 mLInitial pressure (P₁) = 655 mmHGNew volume (V₂) = 125 mLNew pressure (P₂) = ?P₁V₁ = P₂V₂
655 × 325 = P₂ × 125
Divide both sides by 125
P₂ = (655 × 325) / 125
New pressure = 1703 mmHg
11. How do i determine the new volume?The new volume can be obtain as follow:
Initial volume (V₁) = 75 mLInitial pressure (P₁) = 1.50 atmNew pressure (P₂) = 4.5 atmNew volume (V₂) =?P₁V₁ = P₂V₂
1.5 × 75 = 4.5 × V₂
Divide both side by 4.5
V₂ = (1.5 × 75) / 4.5
New volume = 25 mL
12. How do i determine the new volume?The new volume can be obtain as follow:
Initial pressure (P₁) = 760 torrInitial volume (V₁) = 1024 mLNew pressure (P₂) = 115 torrNew volume (V₂) =?P₁V₁ = P₂V₂
760 × 1024 = 115 × V₂
Divide both side by 115
V₂ = (760 × 1024) / 115
New volume = 6767.3 mL
13. How do i determine the pressure in mmHg, torr and atm?i. The pressure in mmHg can be obtain as follow:
Pressure (in psi) = 132 psiPressure (in mmHg) =?1 psi = 51.715 mmHg
Therefore,
132 psi = (132 psi × 51.715 mmHg) / 1 psi
132 psi = 6826.38 mmHg
Thus, the pressure (in mmHg) is 6826.38 mmHg
ii. The pressure in torr can be obtain as follow:
Pressure (in psi) = 132 psiPressure (in torr) =?1 psi = 51.715 torr
Therefore,
132 psi = (132 psi × 51.715 torr) / 1 psi
132 psi = 6826.38 torr
Thus, the pressure (in torr) is 6826.38 torr
iii. The pressure in atm can be obtain as follow:
Pressure (in psi) = 132 psiPressure (in atm) =?14.696 psi = 1 atm
Therefore,
132 psi = (132 psi × 1 atm) / 14.696 psi
132 psi = 8.98 atm
Thus, the pressure (in atm) is 8.98 atm
Learn more about pressure:
https://brainly.com/question/15343985
#SPJ1
Explain why the PbCl2 dissolved when water was added in Step 10. What was the effect of the added water on [Pb2+] and [Ci minus]? In what direction would such a change drive in the reaction? PbCl2(s) = Pb2+(a q) + 2 Ci minus (aq) The concentrations of the ions decreased and the reaction shifted to the right to compensate. The concentrations of the ions increased and the reaction shifted to the left to compensate. The concentrations of the ions decreased and the reaction shifted to the left to compensate. The concentrations of the ions increased and the reaction shifted to the right to compensate
Answers
The \(PbCl_{2}\) dissolved when water was added in Step 10 because the concentrations of the ions decreased and the reaction shifted to the right to compensate.
When water is added to a system in equilibrium, it causes a change in the concentrations of the ions present.
In this case, the addition of water diluted the concentrations of \(Pb^{+2}\) and \(Cl^{-}\) ions, leading to a decrease in their concentrations.
According to Le Chatelier's Principle, when the concentration of the reactants or products changes, the system shifts in the direction that counteracts the change to re-establish equilibrium.
In this case, the decrease in ion concentrations caused the reaction to shift to the right, towards the products, in order to increase the concentrations of the ions and restore equilibrium.
The addition of water to the \(PbCl_{2}\) system caused the concentrations of \(Pb^{+2}\) and \(Cl^{-}\) ions to decrease, leading to a shift in the reaction towards the right to compensate for the change and re-establish equilibrium.
For more information on Le Chatelier's principle kindly visit to
https://brainly.com/question/29009512
#SPJ11
Ground water, the largest source of fresh water, is stored in bodies of rock and or sediment
a. porosity
b. sediment
c. particles
d. aquifers
i dont even know what this means
Answers
Answer:
particles
Explanation:
oof oof oof oof oof oof oof
There are 30.974 grams of phosphorus in one mole. approximately how many atoms of phosphorus are there in one mole? (5.01) 6.02 × 1023 c. 6.02 × 10-23 5.145 × 10-23 d. 1.86 × 1025
Answers
There are approximately 6.02 × 10²³ atoms of phosphorus in one mole of 30.974 grams of phosphorus (option A).
How to calculate number of atoms?The number of atoms of a substance can be calculated by multiplying the number of moles of the substance by Avogadro's number. That is;
no of atoms = no of moles × 6.02 × 10²³
According to this question, there are 30.974 grams of phosphorus in one mole of phosphorus. The number of atoms can be calculated as follows:
no of moles of P = 30.974g ÷ 31g/mol = 0.99mol
number of atoms = 0.99 mol × 6.02 × 10²³
number of atoms = 6.02 × 10²³ atoms
Therefore, there are approximately 6.02 × 10²³ atoms of phosphorus in one mole of 30.974 grams of phosphorus.
Learn more about number of atoms at: https://brainly.com/question/8834373
#SPJ1
the molecular weight of the compund is knwon to be 58.1 g/mol. what is the molecular formula of the compund
Answers
The molecular formula of the compound with a known molecular weight of 58.1 g/mol cannot be determined with just this information.
The molecular weight of a compound can be calculated by summing the atomic weights of all the atoms present in the molecule. However, the molecular weight alone does not provide information about the specific types or numbers of atoms in the compound.
Therefore, more information is needed, such as the elemental composition of the compound, to determine its molecular formula.
To know more about molecular visit:
https://brainly.com/question/156574
#SPJ11
A swimming pool reading reports that chlorine is present at 130 ppm. How many grams of chlorine are present per liter of pool water?
Answers
130 ppm will contain 0.12985167 grams of chlorine present in per liter of pool water.
What is ppm?This is an abbreviation for "parts per million" and it also can be expressed as milligrams per liter (mg/L).
1001.142303 ppm is equal to 1 g/L
So, 130 ppm will contain \(\frac{130}{1001.142303}\) grams of chlorine present in per liter of pool water which is equal to 0.12985167 gram/L.
Learn more about ppm here:
https://brainly.com/question/2618749
#SPJ1
36) What is the mass of a 4.259 g/cm substance which takes up 250.00 cm of space?
Answers
Answer:
The answer is 1064.75 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
volume of substance = 250 cm³
density = 4.259 g/cm³
We have
mass = 4.259 × 250
We have the final answer as
1064.75 gHope this helps you
Which of the following elements has properties different than the rest? (5 points)
H
K
N
O
Answers
Answer:
I think it is K.
Explanation:
It is has more gas than the others.
Answer:
K
Explanation: K(Potassium) is actually a very weak metal that is usually in solid form. All of the other options(Hydrogen, Oxygen, and Nitrogen) are nonmetals that are usually gasses.
Which three of the following statements are true?
-The bilayer of a cellular membrane is primarily composed of amphipathic (amphiphilic) lipids.
-The tendency of hydrophobic molecules to aggregate in water is called the hydrophobic effect.
-Forming an ordered network of water around hydrophobic molecules increases the entropy of water.
-Nonpolar molecules that have no polar groups (e.g. hydrocarbons) can readily form micelles.
-Placing a hydrophobic molecule into water causes water molecules to orient themselves around it.
Answers
The three true statements are:1,2,3
Three true statements from the provided options are:
The bilayer of a cellular membrane is primarily composed of amphipathic (amphiphilic) lipids. This statement is true. The cellular membrane consists of a phospholipid bilayer, with the hydrophilic (polar) heads facing the aqueous environment and the hydrophobic (nonpolar) tails facing inward, creating a barrier.
The tendency of hydrophobic molecules to aggregate in water is called the hydrophobic effect. This statement is true. Hydrophobic molecules repel water due to their nonpolar nature, causing them to aggregate together to minimize contact with water. This phenomenon is known as the hydrophobic effect.
Placing a hydrophobic molecule into water causes water molecules to orient themselves around it. This statement is true. In order to minimize contact with hydrophobic molecules, water molecules arrange themselves in an ordered network around the hydrophobic molecule, forming a hydration shell. This arrangement is energetically favorable and helps to stabilize the system.
The other statements, such as the increase of water entropy around hydrophobic molecules and the formation of micelles by nonpolar molecules, are not true. Increasing the order of water around hydrophobic molecules decreases the entropy of water, and nonpolar molecules typically form micelles in nonpolar solvents rather than in water.
To know more about Nonpolar molecules refer here:
https://brainly.com/question/28300881
#SPJ11
In general the formation of a chemical bond lowers the _____ energy of a chemical system, leading to a _____ stable arrangement.
Answers
In general, the formation of a chemical bond lowers the potential energy of a chemical system, leading to a more stable arrangement.
What is potential energy?Stored energy depends upon the relative position of various parts of a system.
In bond formation, atoms lose their potential energy to attain stability and heat is evolved. The bond formation is an exothermic reaction.
Atoms bond together to form compounds to attain lower energies than they possess as individual atoms.
A quantity of energy is equal to the difference between the energies of the bonded atoms and the energies of the broken atoms.
Learn more about the potential energy here:
https://brainly.com/question/24284560
#SPJ1
Explain the role light plays on the rate
of photosynthesis.
Answers
Answer:
The process of photosynthesis occurs when green plants use the energy of light to convert carbon dioxide (CO2) and water (H2O) into carbohydrates. Light energy is absorbed by chlorophyll, a photosynthetic pigment of the plant, while air containing carbon dioxide and oxygen enters the plant through the leaf stomata.
In the levels of organization of an organism, what does a group of cells make up?
Answers
Answer:
they make tissue. form organ and whole body an i true..
The side of the ladder are made up of alternating ____ and ____ molecules the steps of the ladder are made up of _____ held together by hydrogrn
Answers
The side of the ladder are made up of alternating sugar and phosphate molecules the steps of the ladder are made up of bases held together by hydrogen bonds.
The fundamental unit of DNA is referred to as a nucleotide. One sugar molecule, one phosphate molecule, and one of the four bases make up a nucleotide. DNA's nucleotides align to form two lengthy backbones that resemble a ladder's handrails in the shape of sugar and phosphate molecules. Between the sugar molecules on the two handrails, two bases combine to form the ladder's rungs. The "rungs" between the phosphate molecules are absent. This was significant to Watson and Crick since it enabled them to understand the formation of the double helix.
James Watson and Francis Crick discovered the DNA structure in 1953. A double helix, often known as a twisted ladder, is the structure. Sugar and phosphate molecules alternately make up the sides of the ladder. Hydrogen bonds serve as a flimsy means of holding the two sides of the DNA ladder together.
To know more about the structure of DNA, refer to the following link:
https://brainly.com/question/14537776
#SPJ4
The density of an unknown metal is 8.94 g/cm3 and its atomic radius is 0.126 nm . It has a face-centered cubic lattice. find the atomic mass of this metal
Answers
The atomic mass of the unknown metal is 238 g/mol.
The density of an unknown metal is 8.94 g/cm³ and its atomic radius is 0.126 nm. It has a face-centered cubic lattice. We must first establish a connection between the face-centered cubic (fcc) unit cell and the atomic radius.The fcc unit cell's sides are made up of four atoms, one in each of the cell's corners and one in the center of each face. Thus, the length of the cell's diagonal is 4r/√2, where r is the atomic radius.
We can make a calculation using this information. Let a be the length of one of the sides of the cubic cell, then: a = 2r√2The volume of the unit cell (V) is: V = a³/4 = 4r³/3√2The mass of one unit cell (m) is calculated using the density (ρ):ρ = m/V => m = ρ x V Substituting V from the previous equation:
m = (ρ x 4r³)/3√2
The number of atoms (N) in the unit cell is calculated by dividing the volume of the unit cell by the volume of one atom (V1):N = V/V1 = (a³/4) / ((4πr³)/3) = (a/r)³ / (4π/3) = (4r/√2r)³ / (4π/3) = 16 / 3πThus, the atomic mass (M) of the metal is:M = m/N = (ρ x 4r³)/3√2 x 3π/16 = (ρ x r³ x 4π/4)/√2 x 4 = ρ x r³ x √2/2So,M = 8.94 g/cm³ x (0.126 nm)³ x √2/2 = 238 g/mol Therefore, the atomic mass of the unknown metal is 238 g/mol.
To learn more about atomic mass visit below link
https://brainly.com/question/29117302
#SPJ11
Calculate the pH of a solution that is 0.080 M in trimethylamine, (CH3)3N, and 0.13 Min trimethylammonium chloride, ((CH3)3 NHCI). Express your answer to two decimal places. pH = ____. Part C Calculate the pH of a solution that is made by mixing 50.0 mL of 0.16 M acetic acid and 50.0 mL of 0.21 M sodium acetate. pH = ____.
Answers
The pH of the solution is 4.64.
For the first part of the question:
The reaction that occurs between trimethylamine and trimethylammonium chloride can be written as:
(CH3)3N + H2O ⇌ (CH3)3NH+ + OH-
The equilibrium constant expression for this reaction is:
Kb = [ (CH3)3NH+ ][OH-] / [ (CH3)3N ]
We know the concentration of trimethylamine is 0.080 M and the concentration of trimethylammonium chloride is 0.13 M. Since the two compounds are in equilibrium, we can assume that the concentration of trimethylammonium ion ( (CH3)3NH+ ) is also 0.13 M.
Now we can use the Kb expression to find the concentration of hydroxide ions (OH-) in the solution:
Kb = [ (CH3)3NH+ ][OH-] / [ (CH3)3N ]
5.9 x 10^-5 = (0.13)(OH-) / 0.080
OH- = 1.16 x 10^-4 M
To find the pH, we need to find the concentration of hydrogen ions (H+) in the solution, which can be found using the equation:
Kw = [H+][OH-]
1.0 x 10^-14 = [H+](1.16 x 10^-4)
[H+] = 8.62 x 10^-11 M
pH = -log[H+] = 10.06
Therefore, the pH of the solution is 10.06.
For the second part of the question:
We know that acetic acid (CH3COOH) is a weak acid and sodium acetate (CH3COONa) is a salt of a weak acid and strong base. When the two are mixed together, they will react to form an equilibrium between acetic acid and acetate ion (CH3COO-):
CH3COOH + CH3COO- ⇌ H+ + CH3COO-
The equilibrium constant expression for this reaction is:
Ka = [H+][CH3COO-] / [CH3COOH]
We know the concentrations of acetic acid and sodium acetate, but we need to find the concentrations of hydrogen ions and acetate ions. To do this, we need to use the Henderson-Hasselbalch equation:
pH = pKa + log [base]/[acid]
where pKa is the dissociation constant of acetic acid, which is 4.76.
We can rearrange the equation to solve for [base]/[acid]:
[base]/[acid] = 10^(pH - pKa)
For this solution, we have:
[base]/[acid] = 10^(4.76 - pH)
For the base, we use the concentration of sodium acetate (since it is the salt of a weak acid and strong base, we can assume it completely dissociates in water):
[base] = 0.21 M
For the acid, we use the concentration of acetic acid:
[acid] = 0.16 M
Now we can plug in the values and solve for the pH:
[base]/[acid] = 10^(4.76 - pH)
0.21 / 0.16 = 10^(4.76 - pH)
1.3125 = 10^(4.76 - pH)
log(1.3125) = 4.76 - pH
pH = 4.76 - log(1.3125)
pH = 4.76 - 0.119
pH = 4.64
Learn More about here :-
https://brainly.com/question/30656928
#SPJ11
What is the name of this ??

Answers
Answer:
Explanation:
Your answer is C
Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar) and forms sodium acetate, water, and carbon dioxide gas.
The chemical equation for this reaction is:
NaHCO3 + C2H4O2 → NaC2H3O2 + H2O + CO2
How can you use the chemical equation to explain what you observed when you added vinegar (acetic acid) to baking soda (sodium bicarbonate)? Use atoms in your explanation.
Answers
From the balanced chemical equation of a reaction we can clearly understand the reactants and products in a reaction as well as their stoichiometric ratio in the reaction.
What is a chemical equation ?The chemical equation of a reaction is representing the combination of reactants to form the products. The molecular formula of compounds are used to express them.
Here, the reaction between sodium carbonate and vinegar produces sodium acetate, water and carbon dioxide. The reaction can be easily understood by the evolution of carbon dioxide gases.
So, when we pour vinegar to baking soda, we will observe an effervescence or bubbles of a gas. That gas is carbon dioxide that is clear from the chemical equation.
Find more on chemical equations:
https://brainly.com/question/28294176
#SPJ1
Which of the following best explains the distinctive colors of specific elements when used in
fireworks?
A
the number of electrons in the atoms
B
electrons moving from an excited energy state to the ground state
С
the number of neutrons in the atoms
D
electrons transferring from one atom to a different atom
Answers
The distinctive colors of specific elements when used in fireworks owes to electrons moving from an excited energy state to the ground state.
Neils Bohr in his atomic model postulated that electrons in atoms are found in energy levels. Each energy level corresponds to a fixed amount of energy.
The lowest energy level in the atom is called the ground state and higher energy levels are called excited states.
When an electron receives energy, it can move from a ground state to excited state.
This excess energy is given out as visible light of a characteristic wavelength or color when the electron is moving from an excited energy state to the ground state.
This accounts for the distinctive colors of specific elements when used in
fireworks.
Learn more: https://brainly.com/question/1596638
i just need #2, its due any minute. giving extra points, and will mark brainliest!!!!!!!!
which unknown has the weakest attractive forces?
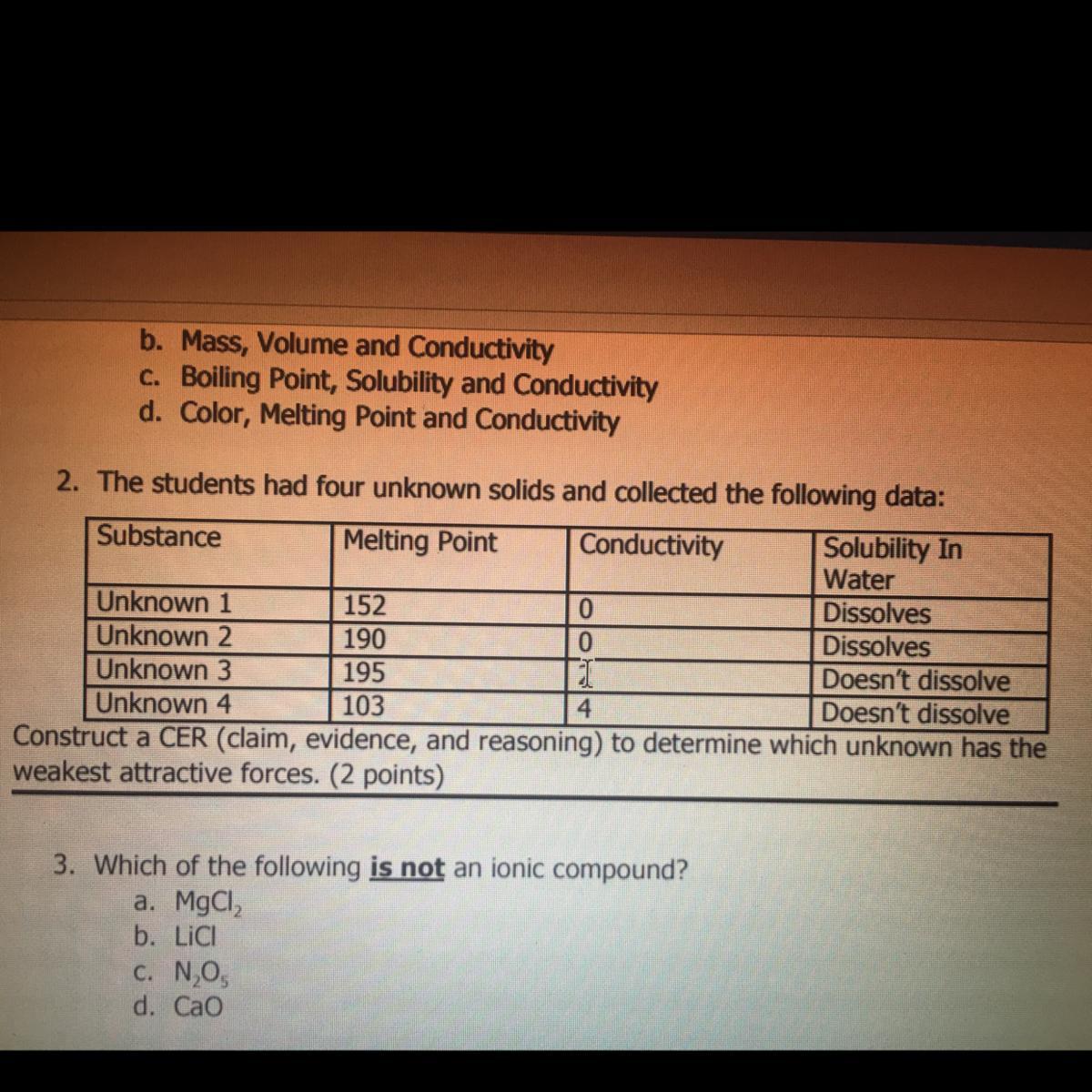
Answers
Answer:
Unknown 4
Explanation:
the number of hydrogen bonds between complementary g–c pairs is _______.
Answers
The number of hydrogen bonds between complementary G-C (guanine-cytosine) pairs is three.
In DNA, hydrogen bonding plays a crucial role in maintaining the stability and structure of the double helix. Guanine (G) and cytosine (C) are complementary base pairs, meaning they form specific hydrogen bond interactions with each other.
Guanine contains a hydrogen bond donor site (NH) and a hydrogen bond acceptor site (O) on its base, while cytosine has a hydrogen bond acceptor site (N) and a hydrogen bond donor site (H). These complementary functional groups allow for the formation of three hydrogen bonds between G and C.
One hydrogen bond forms between the NH group of guanine and the acceptor site of cytosine, while two hydrogen bonds form between the acceptor site of guanine and the NH and H groups of cytosine. These hydrogen bonds provide strength to the G-C base pairing and contribute to the overall stability of the DNA double helix structure.
To know more about the complementary functional groups refer here :
https://brainly.com/question/1356508#
#SPJ11
what alkene is required to synthesize the following compound? a three-membered ring, where the first and the second (clockwise) atoms are carbons and the third atom is an o atom. an h atom is attached to the first atom with a wedge and to the second atom with a dash. a ch2ch2ch3 group is attached to the first atom with a dash. a ch2ch3 group is attached to the second atom with a wedge. draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. the single bond is active by default. show the appropriate stereochemistry by choosing the dashed or wedged buttons and then clicking a bond on the canvas.
Answers
To synthesize the compound described, the required alkene is 2-methyl-3-butene.
This can be drawn on the canvas using the tools, atoms, and advanced template toolbars. To draw the molecule, add two carbon atoms to the canvas and then add a third atom, an oxygen atom, as the third atom in the three-membered ring. To add the hydrogen atoms, use the wedge and dash buttons to attach the hydrogen atom to the first and second carbon atoms respectively.
Use the dash button to attach the CH2CH2CH3 group to the first carbon atom, and use the wedge button to attach the CH2CH3 group to the second carbon atom. Finally, to show the appropriate stereochemistry, use the dashed or wedged buttons and then click a bond on the canvas.
Learn more about stereochemistry at https://brainly.com/question/28658912
#SPJ11