Answers
Answer:
to find out how somethings work
Explanation:
Answer:
Doing experiments is good because when you try these possibilities you can learn about something that you've tried or see why this experiment didn't really work. It also helps you understand that not everything you try will work. that's why we experiment.
Explanation:
Hope this helps:)
Related Questions
Drag the tiles to the correct locations on the equation. Not all tiles will be used.
Two atoms interact with each other and change as shown by the equation. Complete the equation by filling in the missing parts.
5
2
4
3
1
H+H -
H
He
Li
+
Answers
The equation in the question is: H+H → H + H Complete the equation by filling in the missing parts. missing part is 1 → H+H-2 → →3 → He.
The atomic number of hydrogen is 1, which means it has only one proton in the nucleus and one electron in its shell. Two hydrogen atoms react with each other to form helium. Helium has 2 protons and 2 neutrons in its nucleus and two electrons in its shell. Therefore, the equation is:
H + H → HeIt can be seen that:1. H + H (Reactants)
2. → (Yields or Reacts to form)
3. He (Product)Therefore, the tiles will be arranged as shown below: 1 → H+H-2 → →3 → He
For more question atomic number
https://brainly.com/question/16858932
#SPJ8
Jane is sitting in the family car. Her mother is driving her from their house to the library. Jane waves as she passes her friend Marina. Which of the following is not moving with respect to Jane?
A. Marina
B. The family car
C.The library
D. Jane’s house
Answers
Answer:
B) The family car
Explanation:
I hope this helps :)
A substance is considered to have a smaller surface area when...
A. it is a fine powder
B. it is in large chunks
C. you have a small amount of
the substance
D. you have a large amount of E. the substance
Answers
Use the information in the table below to respond to the statements and questions that follow. Your answers should be in terms of principles of molecular structure and intermolecular forces. a) Draw the complete Lewis electron-dot diagram for ethyne in the appropriate cell in the table below. (1) Compound Ethanethiol Formula CH3CH2SH Ethane CH3CH3 Lewis Electron Dot Diagram HH H:C:C:S:H HH HH H:C:C:H HH HH H:C:C:0:H HH Ethanol CH3CH2OH Ethene CzH b) Which of the four molecules contains the shortest carbon-to-carbon bond? Explain. [2] c) Explain how the structure of the molecules affects the energy that is required to boil ethanol. Consider the statement " As ethanol boils energy goes into breaking C-C bonds, C-H bonds, C-o bonds and O-H bonds." Is the statement true or false? Justify your answer. [2] d) Ethanol is completely soluble in water, whereas ethanethiol has limited (significantly less) solubility in water. Account for the difference in solubilities between the two compounds in terms of intermolecular forces. [2]
Answers
The complete Lewis electron-dot diagram for ethyne is shown in the attached diagram below:
What is the lewis electron dot diagram?A lewis electron dot structure can be utilized to represent the number of bonds, the lone pairs left in the atoms, and the bonding atoms in the molecule.
Solid lines are used to represent the bonds between atoms that are directly bonded to one another and excess electrons are denoted as dot pairs and are represented next to the atoms.
As the valence electrons of each carbon atom are equal to 4 from the electronic configuration of the carbon atom. First, the total number of valence electrons in a molecule is 4 + 1 + 1 + 4 = 10.
As each carbon atom needs only 4 electrons to complete its octet. As the octet completes, the rest of the electrons are assigned as the lone pairs of atoms. But there are no lone pairs in the molecule of the ethyne.
Learn more about the lewis dot diagram, here:
brainly.com/question/14091821
#SPJ4

How many atoms are there in 46.4 g of sulfur?
Answers
Answer:
371.2
Explanation:
In the Periodic Table, which of the highlighted elements has the greatest number of protons?
A Fluorine, F
B Iron, Fe
C Calcium, Ca
D Aluminum, AI

Answers
Answer:
since the modern periodic table is made in ascending order by the atomic number fluorine has the most protons.
The highlighted element that has the greatest number of protons is Iron, Fe. Thus, the correct option for this question is B.
What do you mean by Protons?Protons may be defined as a type of subatomic particles which are generally present in the centermost part of an atom known as the nucleus along with neutrons. These subatomic particles are positively charged in nature. Ernest Rutherford was known to discover these particles.
According to the question, the number of protons is generally governed by an atomic number of a chemical element. For example, fluorine has an atomic number of 9. It has the same number of protons in its nucleus. The number of protons in Iron is 26, while the number of protons in Calcium is 20. And the number of protons in aluminum is 13.
Therefore, an iron is the highlighted element that has the greatest number of protons. Thus, the correct option for this question is B.
To learn more about Protons and atomic numbers, refer to the link:
https://brainly.com/question/1805828
#SPJ2
How many kilojoules of heat are needed to raise the temperature of 10g of aluminum from 22 degrees C to 55 degrees C, if the specific heat of aluminum is .901 j/gc?
Answers
Answer:
name four agricultural inputs are subsidized by the government
0.297 kJ of heat is needed to raise the temperature of 10g of aluminum from 22 degrees Celsius to 55 degrees Celsius.
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
It is a measure of how much energy it takes to raise the temperature of a substance. It is the amount of heat necessary to raise one mass unit of that substance by one temperature unit.
It is given by the formula -
Q = mcΔT
where, Q = amount of heat
m = mass
c = specific heat
ΔT = Change in temperature
Given,
mass = 10g
c = 0.901J/g⁰C
Initial temperature (T₁) = 22⁰C
Final Temperature (T₂) = 55⁰C
Q = mcΔT
= 10 × 0.901 × (55 -22)
= 297.33 J = 0.297 kJ
Learn more about Specific heat, here:
https://brainly.com/question/31608647
#SPJ1
what is chemistry...
jab-njko-rzv
Answers
Answer:
Chemistry is the science of matter, its properties, structure, changes or reactions and the energy that accompanies these changes.
Hello,
After doing the Knewton assignments and watching some videos. I really do not understand some basics concepts of Energy. One of the questions is asking: Which of the following objects has more kinetic energy than does potential energy?
a) a compressed spring in a pinball machine just before the ball is launched.
b)a rollercoaster motionless at the top of the track.
c) a yo-yo before it is released
d)the pendulum of a grandfather clock at the lowest point of its arc of motion.
I thought that the answer was b, but the correct answer was c). I did not understand the explanation. It says " Kinetic energy is the energy of motion. Each of the objects above are motionless—with stored potential energy—except for the clock. At the bottom of its arc it will have the greatest kinetic energy, which is then converted to potential energy as it swings back up to one side"
So if someone could explain this better I would so good.
Answers
Answer both of these true or false correctly
brainliest if correct

Answers
Answer:
There both true.
Explanation:
Hope this helps
Answer:
The first one is True
The second one is false
Explanation:
A 4.00L flask containing Ne at 25 C and 6.00 atm is joined by a valve to an 8.00 L flask Ar at 25 C and 2.00 atm.
The valve is opened and the gases mix. If the temperature is constant, what is the (total) pressure in the connected flasks after mixing?
answer: ? atm
Answers
Answer:
\(P=3.33atm\)
Explanation:
Hello!
In this case, since know the volume, temperature and pressure of the initial containers, we can compute the moles of each gas prior to the opening of the valve as shown below:
\(n_{Ne}=\frac{6atm*4L}{0.08206\frac{atm*L}{mol*K}*298.15K} =0.981molNe\\\\n_{Ar}=\frac{2atm*8L}{0.08206\frac{atm*L}{mol*K}*298.15K} =0.654molAr\)
Next, we add them up to obtain the total moles:
\(n_T=0.981mol+0.654mol=1.635mol\)
Now, the total volume:
\(V_T=4.00L+8.00L=12.00L\)
Finally, the total pressure is computed by using the ideal gas equation:
\(P=\frac{1.635mol*0.08206\frac{atm*L}{mol*K}*298.15K}{12.00L}\\\\P=3.33atm\)
Best regards!
aqueous lead (ii) chloride reacts with aqueous sodium sulfate to produce a lead(ii) sulfate precipitate and aqueous sodium chloride
Answers
The equation of the reaction leading to the formation of the precipitate is; \(PbCl_{2}(aq) + Na_{2} SO_{4} (aq) ---- > PbSO_{4}(s) + 2NaCl(aq)\)
What is the precipitate?We define a precipitate as a compound that can be formed as a solid when we mix two aqueous solutions. An aqueous solution is a solution of a substance that have been dissolved in water.
Thus, when we have a mixture of two liquid reactants and then one of the products does separate itself out of the solution then we say that a precipitate has been formed.
In this case, we are to write down the equation of the reaction between aqueous lead (ii) chloride reacts with aqueous sodium sulfate to produce a lead(ii) sulfate. The solid would separate out of the system and we can see the white color of the lead(ii) sulfate.
Learn more about precipitate:https://brainly.com/question/16950193
#SPJ1
CAN SOMEONE HELP WITH THESE QUESTIONS BASED ON THE TYPES OF CHEMICAL REACTIONS EXPERIMENT?✨
1- What type of chemical reaction takes place when magnesium metal is added to a solution of iron(ll) sulfate?
2- Which of the compounds or ions used in this lab is toxic?
3- What is a catalyst?
4- Why is universal indicator in this lab?
Answers
The type of chemical reaction takes place when magnesium metal is added to a solution of iron(ll) sulfate is displacement reaction.
Magnesium sulfate in large doses may be toxic.
A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction.
A universal indicator is a pH indicator made of a solution of several compounds that exhibits several smooth colour changes over a wide range pH values
What is Displacement reaction?A displacement reaction is a type of reaction in which part of one reactant is replaced by another reactant. A displacement reaction is also known as a replacement reaction or a metathesis reaction.
Single displacement reactions are reactions where one reactant replaces part of the other:
AB + C → AC + B
An example is the reaction between iron and magnesium sulfate to produce iron sulfate and copper:
Fe + MgSO₄ → FeSO₄ + Mg
Therefore,
The type of chemical reaction takes place when magnesium metal is added to a solution of iron(ll) sulfate is displacement reaction.
Magnesium sulfate in large doses may be toxic.
A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction.
A universal indicator is a pH indicator made of a solution of several compounds that exhibits several smooth colour changes over a wide range pH values
Learn more about Displacement reaction, here:
https://brainly.com/question/29307794
#SPJ1
It is difficult to break the ionic bonds in a compound because of the
Answers
A filled balloon has a volume of 5.6 L at 35 ˚C and it is placed into liquid nitrogen at a temperature of -78 ˚C. What is the new volume of the balloon?
Answers
Answer:
3.54 L
Explanation:
To find the new volume, we'll use this formula: \(\frac{V_1}{T_1} = \frac{V_2}{T_2}\)
V₁ = Liters (5.6)
T₁ = Volume in kelvin (273 + 35 = 308)
V₂ = New liters (?)
T₂ = New temperature (273 - 78 = 195)
\(\frac{5.6}{308} = \frac{V_2}{195}\)
First, cross multiply
1092 = V₂308
Divide both sides by 308
1092/308 = V₂308/308
V₂ = 3.54
Chlorine is made up of 75.78% 35cl and 24.22% 37 cl. The atomic mass of 35 CL is 34.969 amu. The atomic mass of 37 CL is 36.966 amu. What is the average atomic mass of a sample of chlorine?
Answers
35.453 amu is the correct answer
1. Calculate the molarity of a barium hydroxide solution if you used 41.65 mL of it to neutralize 1.190 g of potassium hydrogen phthalate. Ba(OH) 2 (aq) 2 KHC 8 H 4 O 4 (aq) BaC 8 H 4 O 4 (aq) K 2 C 8 H 4 O 4 (aq) 2 H 2 O(l) 2. Write a balanced chemical equation for the reaction of hydrochloric acid with sodium hydroxide. 3. Calculate the molarity of a hydrochloric acid solution if 34.21 mL of 0.0431M sodium hydroxide neutralizes 25.00 mL of the acid solution. 4. Write a balanced chemical equation for the reaction of phosphoric acid with sodium hydroxide. 5. Calculate the molarity of a phosphoric acid solution if 34.21 mL of 0.0431M sodium hydroxide neutralizes 25.00 mL of the acid solution.
Answers
Answer:
1. 0.0700M barium hydroxide.
2. HCl + NaOH → H₂O + NaCl
3. 0.0590M HCl
4. H₃PO₄ + 3NaOH → Na₃PO₄ + 3H₂O
5. 0.01967M H₃PO₄
Explanation:
1. Based on the reaction:
Ba(OH)₂(aq) + 2KHC₈H₄O₄(aq) → BaC₈H₄O₄(aq) + K₂C₈H₄O₄(aq) + 2H₂O(l)
To find the molarity we have to convert the KHP to moles. With moles and volume we can find the molarity of the basrium hydroxide as follows:
Moles KHP -Molar mass: 204.22g/mol-
1.190g * (1mol / 204.22g) = 5.827x10⁻³ moles KHP
Moles Ba(OH)₂:
5.827x10⁻³ moles KHP * (1mol Ba(OH)₂ / 2mol KHP) = 2.91x10⁻³ mol Ba(OH)₂
Molarity:
2.91x10⁻³ mol Ba(OH)₂ / 0.04165L =
0.0700M barium hydroxide2. An acid reacts with a base to produce water and the salt. When HCl, reacts with NaOH, the reaction is:
HCl + NaOH → H₂O + NaCl3. The moles of NaOH added are:
34.21mL = 0.03421L * (0.0431mol / L) = 1.474x10⁻³ moles NaOH = Moles HCl
The molarity is:
1.474x10⁻³ moles HCl / 0.02500L =
0.0590M HCl4. The phosphoric acid, H₃PO₄, reacts with NaOH as follows:
H₃PO₄ + 3NaOH → Na₃PO₄ + 3H₂O5. The moles of NaOH added are:
34.21mL = 0.03421L * (0.0431mol / L) = 1.474x10⁻³ moles NaOH
The moles of phosphoric acid are:
1.474x10⁻³ moles NaOH * (1mol H₃PO₄ / 3mol NaOH) =
4.91x10⁻⁴ moles H₃PO₄. In 25.00mL = 0.02500L:
4.91x10⁻⁴ moles H₃PO₄ / 0.02500L =
0.01967M H₃PO₄31.7 grams of water form based on the following equation. What was the change in heat
for the reaction?
CH4 + 2 O2 → CO₂ + 2 H₂O
ΔΗ = -890.8 kJ/mol
Answers
31.7 g of water formation in the given reaction releases 783.02 kJ of heat energy.
The change in heat in the given equation is -890.8 kJ/mol. This means that when one mole of CH4 reacts with 2 moles of O2, it produces one mole of CO2 and 2 moles of H2O while releasing 890.8 kJ of heat energy.Now, we have to find out how much heat energy will be released when 31.7 g of water is formed. To do this, we need to first calculate the number of moles of water formed from 31.7 g of H2O.Molar mass of H2O = 2 × 1.008 + 15.999 = 18.015 g/molNumber of moles of H2O = 31.7 g / 18.015 g/mol = 1.759 molNow we know that 2 moles of H2O are formed when 1 mole of CH4 reacts. Therefore, the number of moles of CH4 required to produce 1.759 mol of H2O will be:1 mole of CH4 : 2 moles of H2Ox moles of CH4 : 1.759 moles of H2Ox = 1.759/2 = 0.8795 molSo, 0.8795 moles of CH4 are required to produce 1.759 moles of H2O. And the heat released during the reaction of 0.8795 mol CH4 can be calculated using the given change in heat.ΔH = -890.8 kJ/molHeat released during the reaction of 0.8795 mol CH4= ΔH × number of moles= -890.8 kJ/mol × 0.8795 mol= -783.02 kJ.
for more questions on heat
https://brainly.com/question/30738335
#SPJ8
A metal, M , of atomic mass 56 amu reacts with chlorine to form a salt that can be represented as MClx. A boiling point elevation experiment is performed to determine the subscript , and therefore, the formula of the salt. A 30.2 g sample of the salt is dissolved in 100.0 g of water and the boiling point of the solution is found to be 376.81 K. Find the formula of the salt. Assume complete dissociation of the salt in solution.
CAN SOMEONE PLEASE HELP ME!!! I AM VERY CONFUSED AND ITS DUE TODAY BEFORE 11:55 THANK YOU IN ADVANCED
Answers
Answer:
MCl₂
Explanation:
The formula for boiling point elevation can be used to find x. The "complete dissociation" means there will be an ion of M and x ions of Cl in the solution. The number of moles of solute will be 30.2 grams divided by the molecular weight of MClx, where x is the variable we're trying to find.
\(\Delta T=imK_b\qquad\text{where i=ions/mole, m=molality, $K_b\approx 0.512$}\\\\376.81-373.15=(x+1)\dfrac{\text{moles}}{\text{kg solvent}}(0.512)\\\\\dfrac{3.66}{0.512}=(x+1)\dfrac{\dfrac{30.2}{56+35.45x}}{0.1}=\dfrac{302(x+1)}{56+35.45x}\\\\\dfrac{3.66}{0.512\cdot 302}(56+35.45x)=x+1\\\\\dfrac{3.66\cdot 56}{0.512\cdot 302}-1=x\left(1-\dfrac{3.66\cdot 35.45}{0.512\cdot 302}\right)\\\\x=\dfrac{50.336}{24.877}\approx 2.023\)
Then the formula for the salt is MCl₂.
How would I use the word evolution in a sentence?
Answers
18 grams of pentane go through a burning reaction, where oxygen is leftover. what would be the volume of the oxygen needed for its entire burning process in normal conditions? ArC=12, ArNa=23, ArH=1, ArO=16
Answers
The balanced equation of the reaction is:C5H12 + 8O2 → 5CO2 + 6H2OFrom the equation, it shows that 8 moles of oxygen is needed to burn 1 mole of pentane. The volume of oxygen needed for the entire burning process of 18 grams of pentane in normal conditions is 44.86 L.
First, let's convert 18 grams of pentane to moles using the molar mass of pentane (C5H12) as follows:Mass of pentane = 18 g Molar mass of pentane (C5H12) = 5(12) + 12(1) = 72 g/molNumber of moles of pentane = Mass/Molar mass = 18/72 = 0.25 moles
Now, to find the volume of oxygen needed for its entire burning process in normal conditions, we'll use the Ideal Gas Law equation as follows: n = PV/RT where :n = number of moles of the gas V = volume of the gas (in liters)P = pressure of the gas (in atmospheres)R = Universal Gas Constant = 0.0821 L· atm /K· molT = temperature of the gas (in Kelvin)From the equation, we know that 8 moles of oxygen are needed to burn 1 mole of pentane.
Therefore, the number of moles of oxygen needed to burn 0.25 moles of pentane will be: n = 8 × 0.25 = 2 moles The temperature and pressure are normal conditions, which is 0°C and 1 atm respectively. Let's convert the temperature to Kelvin:T = 0 + 273 = 273 K Substituting the values into the Ideal Gas Law equation gives: P × V = nRT Rearranging the equation gives: V = nRT/P Substituting the values, we get: V = 2 × 0.0821 × 273/1V = 44.86 L
Therefore, the volume of oxygen needed for the entire burning process of 18 grams of pentane in normal conditions is 44.86 L.
For more such questions on oxygen
https://brainly.com/question/26073928
#SPJ8
In a chemical reaction, a catalyst changes the ______.
Select one:
a.
heat of reaction
b.
activation energy of the reaction
c.
potential energy of the products
d.
potential energy of the reactants
Answers
Answer:
The Correct answer is B
activation energy of the reaction
or
speeds up the reaction
The reaction A→B is to be carried out isothermally in a continuous-flow reactor. Calculate the PFR volume to consume 99% of A (CA = 0.01CA0) when the entering molar flow rate is 5 mol/h (assume pure A), the volumetric flow rate is constant at 10 dm3 /h and the rate is: -rA=3CA 2 [ dm3 /mol•h]
Answers
Answer:
Explanation:
From the information above:
The equation for the reactor volume of CSTR is given by the formula:
\(V = \dfrac{C_{Ao}V_o - C_A V_o }{-rA}\)
\(V = \dfrac{C_{Ao}V_o (1 - 0.01)}{KC_A^2}\)
The reaction rate of A is:
\(-r_A = kC_A^2\) and k = 3 dm³/mol.h
replacing the values in the above equation to solve for v;
∴
\(V = \dfrac{(0.5 \ mol/dm^3) (10 \ dm^3/h) (1 - 0.01)}{ ( 3 \ dm^3/mol.h) (0.01 \times 0.5 \ mol/dm^3)^2}\)
V = 66,000 dm³
Thus, the reactor volume of CSTR is 66000 dm³
Taking the differential mole balance equation for PFR is as follows:
\(\dfrac{d(C_AV_o)}{dV} = -r_A\)
\(\dfrac{d(C_AV_o)}{dV} = Kc^2A\)
Taking the integral between initial and final concentrations;
\(\dfrac{v_o}{k} \int ^{C_A}_{C_{Ao}} \dfrac{dC_A}{C^2A}= - \int ^V_o \ dV\)
\(V = \dfrac{V_o}{K} \Big [\dfrac{1}{C_A}- \dfrac{1}{C_{Ao}} \Big ]\)
replace the values in the above equation;
\(V= \dfrac{(10 \ dm^3/h) }{(3 \ dm^3/mol.h)}\Big [ \dfrac{1}{0.01(0.5 \ mol/dm^3)} - \dfrac{1}{0.5 \ mol/dm^3} \Big ]\)
\(\mathbf{V = 66 0 \ dm^3}\)
Thus, the reactor volume of PFR is 660 dm³
6. How many moles of water would require 92.048 kJ of heat to raise its temperature from 34.0 °C to 100.0 °C? (3 marks)
Answers
Taking into account the definition of calorimetry, 0.0185 moles of water are required.
CalorimetryCalorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
Sensible heat is defined as the amount of heat that a body absorbs or releases without any changes in its physical state (phase change).
So, the equation that allows to calculate heat exchanges is:
Q = c× m× ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
Mass of water requiredIn this case, you know:
Heat= 92.048 kJMass of water = ?Initial temperature of water= 34 ºCFinal temperature of water= 100 ºCSpecific heat of water = 4.186 \(\frac{J}{gC}\)Replacing in the expression to calculate heat exchanges:
92.048 kJ = 4.186 \(\frac{J}{gC}\)× m× (100 °C -34 °C)
92.048 kJ = 4.186 \(\frac{J}{gC}\)× m× 66 °C
m= 92.048 kJ ÷ (4.186 \(\frac{J}{gC}\)× 66 °C)
m= 0.333 grams
Moles of water requiredBeing the molar mass of water 18 \(\frac{g}{mole}\), that is, the amount of mass that a substance contains in one mole, the moles of water required can be calculated as:
\(amount of moles=0.333 gramsx\frac{1 mole}{18 grams}\)
amount of moles= 0.0185 moles
Finally, 0.0185 moles of water are required.
Learn more about calorimetry:
brainly.com/question/11586486?referrer=searchResults
brainly.com/question/24724338?referrer=searchResults
brainly.com/question/14057615?referrer=searchResults
brainly.com/question/24988785?referrer=searchResults
brainly.com/question/21315372?referrer=searchResults
brainly.com/question/13959344?referrer=searchResults
brainly.com/question/14309811?referrer=searchResults
brainly.com/question/23578297?referrer=searchResults
Please help me
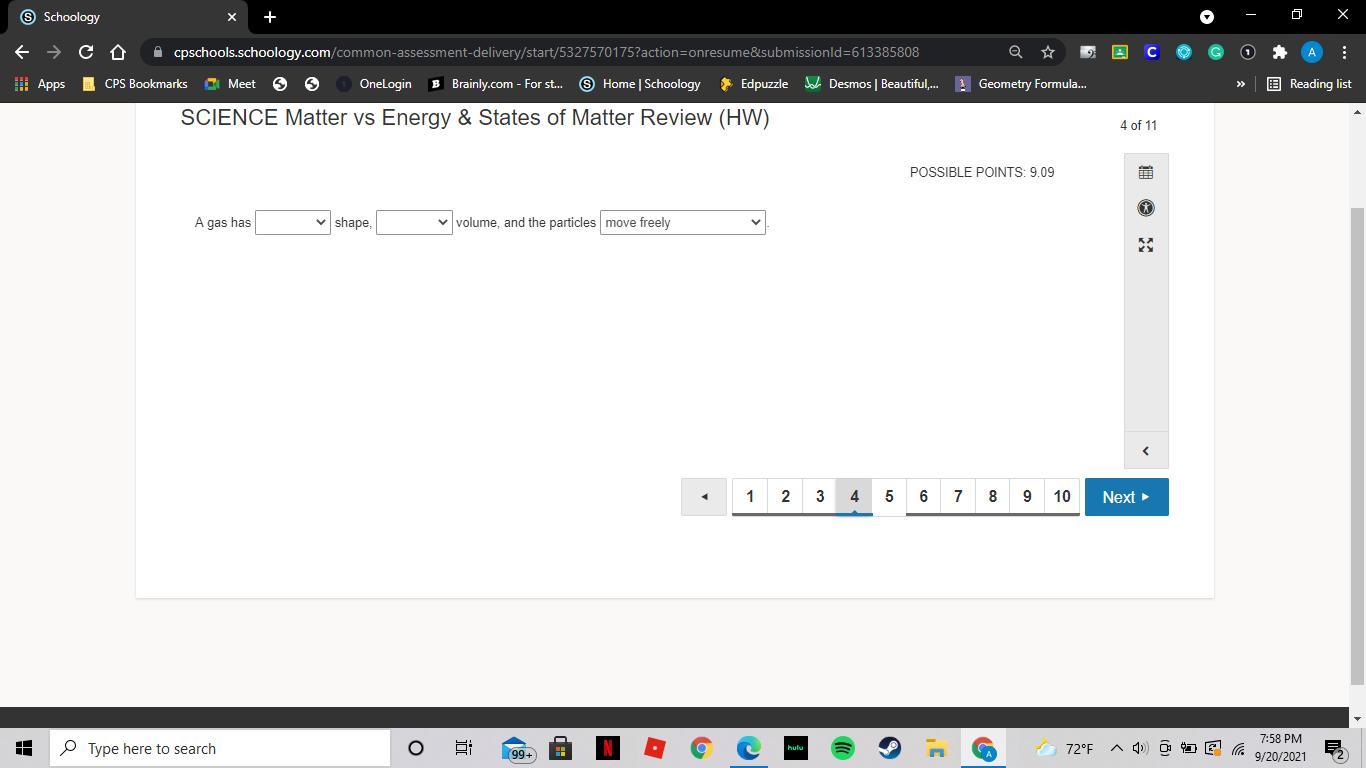
A gas has definite shape or indefinite shape
A gas has indefinite volume or definite volume?
Answers
Identify the limiting reactant in the reaction of methane (CH4) and carbon tetrachloride to form CH2Cl2, if 2.96 g of CH4 and 32.0 g of CCl4 are combined. Determine the amount (in grams) of excess reactant that remains after the reaction is complete.
Answers
Answer:
d
Explanation:
yes
What is the definition of a reducing agent?
Answers
Answer:
a substance that tends to bring about reduction by being oxidized and losing electrons.
Answer:
a substance that reduces a chemical compound usually by donating electrons.
Compute for the pH of 0.415 HCl acid
Answers
Carbon monoxide (CO) and hydrogen (H2) are fed to a continuous catalytic reactor operating at steady state. There are no other components in the feed. The outlet stream contains unconverted CO and H2, along with the products methanol (CH30H), ethanol (C2HsOH), isopropanol (C3H70H), and carbon dioxide (C02). These are the only species in the product stream.
The reactions occuring are:
CO+ 2H2 → CH3OH
3CO+ 3H2 → C2H5OH+ CO2
5CO+ 4H2 → C3H7OH + 2CO2
The feed rates of CO and H2 to the reactor are 100 mol/h(each). the rates in the stream that leaves the reactor (in mols/h) are H2-30; CO-30; C2H5OH-5. What’s the mole fraction of each species in the product steam?
Answers
Answer:
Explanation:
Let the products in the outlet streams be P(i.e. CH₃OH), Q(i.e. C₂H₅OH) and R(i.e. C₃H₇OH) respectively
Then the mass balance for CO, H and C₂H₅OH can be computed as follows:
For CO, The mass balance is;
100 - P - 3Q - 5R = 30 --- (1)
For H₂, The mass balance is;
100 - 2P - 3Q - 4R = 30 --- (2)
For C₂H₅OH, The mass balance is;
Q = 5 --- (3)
Replacing the value of equation(3) into equation (1) and (2); we have:
From equation (1):
100 - P - 3(5) - 5R = 30 --- (1)
100 - 15 - P - 5R = 30
85 - P - 5R = 30
85 - 30 = P + 5R
P + 5R = 55 ----- (4)
From equation (2):
100 - 2P - 3Q - 4R = 30 --- (2)
100 - 2P - 3(5) - 4R = 30
100 - 15 - 30 = 2P +4R
55 = 2P + 4R
2P + 4R = 55 ----- (5)
From equation (4) and (5); we have:
P + 5R = 55 ----- (4)
2P + 4R = 55 ----- (5)
From equation (4);
Let P = 55 - 5R
Then, replace P = 55 - 5R in equation (5)
2(55 - 5R) + 4R = 55
110 - 10R + 4R = 55
110 - 6R = 55
6R = 110 - 55
6R = 55
R = 55/6
R = 9.17
Substitute, the value of R in equation (5); we have:
2P + 4R = 55
2P + 4(9.17) = 55
2P = 55 - 4(9.17)
2P = 55 - 36.68
2P = 18.32
P = 18.32/2
P = 9.16
Therefore, the outlet stream rates are as follows:
Mass Feed Rate ( mol/hr) Molar ratio
CO 30 0.281
H₂ 30 0.281
CH₃OH 9.16 0.085
C₂H₅OH 5 0.047
C₃H₇OH 9.17 0.086
CO₂ 23.34 0.219
Total: 106.67
Choose the equation below that is balanced correctly.
S8 +24 028 SO3
S8+ 12 0₂8 SO3
6 S8+8 026 SO3
2 S8 +3 022 SO3
Answers
The balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is 2S₈ + 16O₂ → 16SO₃.
What is the balanced chemical equation?Balancing chemical equations involves the addition of stoichiometric coefficients to the reactants and products.
The balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is determined as;
2S₈ + 16O₂ → 16SO₃
From the reactants side we can see that sulfur is 16 and also 16 in the product side. The number of oxygen in the reactant side is 32 and also 32 in the product side.
Thus, the balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is 2S₈ + 16O₂ → 16SO₃.
Learn more about balanced chemical equation here: https://brainly.com/question/26694427
#SPJ1