What is the ph of a solution formed by adding 4.3 x 10-5 moles of koh in one liter of water? question 22 options: 2.3 x 10-10 4.4 7.0 9.6 4.3 x 10-5
Answers
The pH of the solution formed by adding 4.3 * 10^-5 moles of KOH in one litre of water is 9.6.
Given:
No. of moles of KOH = 4.3 * 10^-5 moles
Volume of solution = 1L
Molarity = No. of moles / Volume of solution
M = 4.3 * 10^-5 / 1
M = 4.3 * 10^-5
p[OH] = -log[OH^-]
p[OH] + p[H] = 14
pH = 14 - pOH
pOH = 14 - pH
14 - pH = -log(4.3 * 10^-5)
14 - pH = 4.3
pH = 14 - 4.3
pH = 9.6
to learn more about pH click the given link
https://brainly.com/question/26424076
#SPJ4
Related Questions
water is stored where until it is used by the cell
Answers
Answer: The water Inside the cell is stored inside the vacuole(s)
Explanation:
Hope this helps :)
2. A copper wire can conduct electricity whether the wire is
very thin or very thick. Is electrical conductivity an
extensive property or an intensive property?
Answers
Answer:
Intensive
Explanation:
The electrical conductivity of a wire depends on its composition, not the length of the wire.
Hope this helps! If correct pls mark brainliest :)
As copper wire was very thin, it will have great electrical conductance and copper wire has a intensive property.
What is electrical resistance of wire and how copper wire has intensive property?The electrical resistance of a wire is given by
R = ρl/A.
where,
R -Resistivity of the material,
l - length of the wire,
A - cross-sectional area of the wire
As we know the formula, it can be explained as:
The resistance is proportional to the length of the wire.The resistance is inversely proportional to the cross-sectional area. If the resistance is less then it means that it has the cross - sectional will be large and if the resistance is higher then the cross - sectional area will be small.Thick wires has larger cross sectional area and thin wires has small cross sectional area.Hence, we came to know that, thin wires have a greater electrical resistance than a thick wire.
So, copper wire was very thin and it can conduct electricity.
An intensive property is a property of matter that depends on the type of matter of the sample not considered by the amount of matter. Other intensive properties include color, temperature, density, and solubility.Here, concluded that copper wire was very thin and it has intensive property.
Learn more about electrical conductivity of thin wire,
https://brainly.com/question/14074813
#SPJ2
a buffer with a ph of 9.85 contains ch3nh2 and ch3nh3cl in water. what can you conclude about the relative concentrations of ch3nh2 and ch3nh3cl in this buffer? for ch3nh2, pkb
Answers
The solution will be a basic buffer.
The pH should be within 1 pH unit of the weak acid's pKa. This means that the acid and base concentrations should not differ by more than 10-fold. A buffer is a solution that can withstand changes in pH due to the addition of acid or base components. It can neutralize small amounts of added acids or bases and keep the pH of the solution relatively stable.
This is important for processes and reactions that require a specific stable pH range. Buffers maintain a stable pH by neutralizing added acids or bases. They are composed of weak acids and their conjugate bases, which exchange protons and hydroxide ions to form water. The Henderson-Hasselbalch equation describes pH as a function of PKA and the ratio of base concentration to acid concentration.
Learn more about The relative concentrations here:-https://brainly.com/question/10838453
#SPJ4
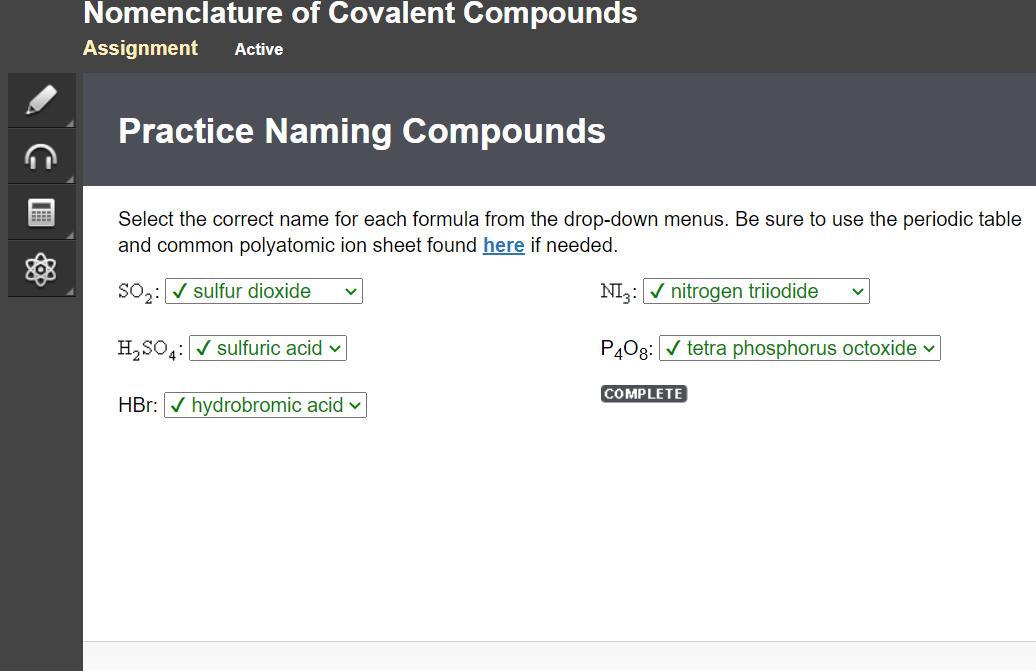
Select the correct name for each formula from the drop-down menus. Be sure to use the periodic table
and common polyatomic ion sheet found here if needed.
Answers
Answer:
here are the answers edge2020
Explanation:

Answer:
Here is 2/5 and the next slide answers too!
Explanation:
Hope it helps!!

which is thought to be more prevalent in the universe: dark matter or dark energy?
Answers
So answer is dark energy;)
Scientists believe that dark matter is more prevalent in the universe than dark energy. Dark matter is estimated to make up approximately 27% of the universe, while dark energy is thought to account for around 68%. The remaining 5% of the universe is made up of visible matter such as stars and galaxies.
Dark matter is a form of matter that does not interact with light or any other electromagnetic radiation, making it invisible to telescopes. It is only detected through its gravitational effects on visible matter. Scientists hypothesize that dark matter plays a crucial role in holding galaxies together and in the large-scale structure of the universe.
On the other hand, dark energy is a mysterious force that is thought to be responsible for the accelerating expansion of the universe. Unlike dark matter, dark energy does not interact with matter at all, and its nature and origin remain largely unknown.
In summary, while both dark matter and dark energy are still not fully understood, scientists believe that dark matter is more prevalent in the universe than dark energy.
Learn more about Dark matter here:
https://brainly.com/question/29265929
#SPJ11
the ability of microbes to utilize n2 as a nitrogen source is called __________.
Answers
The ability of microbes to utilize N2 as a nitrogen source is called Nitrogen fixation.
Nitrogen fixation refers to the process by which certain microorganisms convert inert atmospheric nitrogen (N2) into biologically useful forms of nitrogen. Nitrogen is a crucial component of amino acids, which are building blocks of proteins and nucleic acids (DNA and RNA) essential for life.Nitrogen fixation is classified into two categories: atmospheric and industrial. Nitrogen fixation by industrial processes includes Haber-Bosch and Ostwald processes while biological nitrogen fixation is a natural process. Biological nitrogen fixation is carried out by some free-living soil bacteria, symbiotic bacteria present in the root nodules of leguminous plants, and some other groups of bacteria that live in symbiotic associations with various types of plants.
To learn more about Nitrogen fixation check the link below-
https://brainly.com/question/19972090
#SPJ11
The molar masses and empirical formulas of several compounds containing carbon and chlorine are as listed here. Find the molecular formula of each compound.
Answers
The empirical formula is the simplest formula of a compound. It is the formula of the compound that shows the ratios of each of the atoms that are present in the compound.
Now, let us try to obtain the molecular formula of each compound;
CCl;
284.77 = [12 + 35.5]n
n = 284.77/ [12 + 35.5]
n = 6
Hence the molecular formula is \(C_{6} Cl_{6}\)
\(C_{2} HCl_{3}\);
131.39 = [2(12) + 1 + 3(35.5)]n
131.39 = [24 + 1 + 106.5]n
n = 131.39/[24 + 1 + 106.5]
n = 1
The molecular formula is \(C_{2} HCl_{3}\)
\(C_{2} HCl\);
181.44 = [2(12) + 1 + 35.5]n
181.44 = [24 + 1 +35.5]n
n = 181.44 / [24 + 1 +35.5]
n = 3
The molecular formula is \(C_{6} H_{3} Cl_{3}\)
Learn more about molecular formula:https://brainly.com/question/28647690
#SPJ1

The reaction you will be performing requires a source of chloride ion, which is the required nucleophile. A sodium chloride solution would be much more safe than a hydrochloric acid solution. Why is acid required?.
Answers
Because it provides a very high concentration compared to what is possible with saline.
A high chloride ion concentration shifts the equilibrium position to the right due to Le Chatelier's principle.
Uses of HCL -
Hydrogen Chloride is used in the production of commercial hydrochloric acid. It's HCl, but it's an aqueous solution. It dissociates in water to form hydronium cations and chloride anions. It is a good acidifying agent and is often used as the preferred acid in base number titrations, as stronger acids give more accurate results. Hydrogen chloride has many uses, including cleaning, pickling, electroplating metals, tanning leather, and refining and manufacturing a variety of products.Hydrogen chloride is produced when many plastics are burned. Acute (short-term) inhalation exposure in humans can cause eye, nose, and respiratory tract irritation and inflammation, and pulmonary edema.
To know furthermore about Hydrochloric acid at
https://brainly.com/question/24586675
#SPJ4
Driving automobiles and burning coal for electricity are examples of two human activities that negatively impact _______.
Answers
Answer:
I think its pollution?
Which statement BEST describes the difference between groups and periods in the periodic table of elements?
A) Elements in periods have similar properties, while elements in groups have gradually changing properties
B) Elements in periods have gradually changing chemical properties, while elements in groups have similar properties.
C) Elements in periods are found in vertical columns, while elements in groups are found in horizontal rows.
D) Elements in periods are mostly metals, while elements in groups are mostly metalloids.
Answers
Although elements in grouping have similar properties, those in period have gradually shifting chemical properties.
Describe element.Any compound that cannot be broken down into simpler chemicals by regular chemical processes is referred to as a chemical element or element. The building blocks from which every matter is made are called elements. a molecule that cannot be chemically divided into simpler substances.
What are the four primary chemical elements?About 96% of a human body is made up of just just four substances: carbon (C), oxygenation (O), hydrogen (H), and nitrogen (N). There are 25 identified elements that are necessary for life. According to the required quantity, this graphic divides the important components into three major groupings.
To know more about elements visit:
https://brainly.com/question/24407115
#SPJ1
Answer:
It's B
Explanation:
Trust me, I have 50 million power
The peeling off of outer layers of rock due to temperature changes is called _____.
A exfoliation
B. burrowing
C. frost wedging
D. abrasion
Answers
Answer:
A. Exfoliation.
Answer:
A. exfoliation
Explanation:
HelpPPPP PLEASEES I need in 3 minutes

Answers
ke=0
PLEASE help me with this stoichiometry problem! Picture includedd. 20 Points if you help me! I found the answer by looking it up, but I don't get the steps. Can someone tell me the steps?

Answers
2.5 moles of Al₂O₃ can form from 5.0 moles of Al.
What is mole?In the field of chemistry, moles serve as a fundamental unit of measurement to quantify the amount of a given substance. To be precise, one mole is equivalent to the number of elementary entities - atoms, molecules or ions - present in 12 grams of carbon-12. This definition helps scientists accurately measure and express quantities in their experiments and research endeavors.
Equation:
4 Al + 3 O₂ → 2 Al₂O₃
From the balanced equation, we can see that 4 moles of Al reacts with 3 moles of O₂ to form 2 moles of Al₂O₃.
So, for every 4 moles of Al used in the reaction, 2 moles of Al₂O₃ are produced. We can use this ratio to calculate the moles of Al₂O₃ that can be formed from 5.0 moles of Al:
Moles of Al₂O₃ = (5.0 mol Al) x (2 mol Al₂O₃ / 4 mol Al) = 2.5 mol Al₂O₃
To know more about moles, click here
https://brainly.com/question/26416088
#SPJ1
calculate the standard entropy change for the following reaction at 25°c. ch4(g) 2 o2(g) → co2(g) 2 h2o(l) you must look up the standard entropies of the reactants and products.
Answers
The standard entropy change for the reaction CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(l) at 25°C is -243 J/mol·K.
To calculate the standard entropy change for the following reaction at 25°C: CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(l), you need to look up the standard entropies of the reactants and products.
Here's a step-by-step explanation:
1. Look up the standard entropies (in J/mol·K) of each reactant and product:
- CH4(g): 186.3 J/mol·K
- O2(g): 205.2 J/mol·K
- CO2(g): 213.8 J/mol·K
- H2O(l): 69.95 J/mol·K
2. Multiply the standard entropy of each reactant and product by its stoichiometric coefficient:
- CH4(g): 186.3 J/mol·K × 1 = 186.3 J/mol·K
- O2(g): 205.2 J/mol·K × 2 = 410.4 J/mol·K
- CO2(g): 213.8 J/mol·K × 1 = 213.8 J/mol·K
- H2O(l): 69.95 J/mol·K × 2 = 139.9 J/mol·K
3. Calculate the total entropy of the reactants and the products:
- Total entropy of reactants: 186.3 J/mol·K + 410.4 J/mol·K = 596.7 J/mol·K
- Total entropy of products: 213.8 J/mol·K + 139.9 J/mol·K = 353.7 J/mol·K
4. Subtract the total entropy of reactants from the total entropy of products to find the standard entropy change:
- Standard entropy change: 353.7 J/mol·K - 596.7 J/mol·K = -243 J/mol·K.
To lean more about standard entropy change, visit:
https://brainly.com/question/15022152
#SPJ11
This catastrophic event hit Sumatra in 2004 and again in 2005, destroying huge amounts of property and killing thousands of people. It is an event generated by earthquakes. It is a A) tsunami. B) tornado. C) hurricane. D) water spout.
Answers
Answer: A
Explanation: I hope this helps!
Answer:
A or C. Best to go with A
Explanation:
Hope it works!!
if molecules of hydrogen, nitrogen, oxygen and chlorine have the same kinetic energy which molecule will be moving the fastest?
Answers
If molecules of hydrogen, nitrogen, oxygen, and chlorine have the same kinetic energy hydrogen molecule will move the fastest.
Kinetic energy will be defined as:
K.E = 1/2mv2
In the above-mentioned equation:
K.E = kinetic energy
m = mass of gas molecules
v = velocity of gas molecules
Gases with smaller particle sizes tend to be traveling quicker at a given temperature, which means that they have a greater speed. Kinetic energy enables gas particles to move at different rates, and these speeds are determined by the size of the gas particle. Hydrogen gas particles are the smallest and hence move at a faster rate, followed by nitrogen gas particles, oxygen gas particles, and chlorine gas particles.
You can also learn about kinetic energy from the following question:
https://brainly.com/question/26472013
#SPJ4
if 50,000 j of heat are added to 0.9 kg of water, which has a specific heat of 4,184 j/(kgoc) and is at an initial temperature of 25oc, what is the final temperature of the water (in oc)?
Answers
The answer is 39.56°C.
The amount of heat required to raise the temperature of a substance by one degree Celsius depends on the specific heat of that substance. Specific heat is defined as the amount of heat required to raise the temperature of one unit of mass of a substance by one degree Celsius.
Here, we need to find the final temperature of 0.9 kg of water after 50,000 J of heat are added to it. Also given that water has a specific heat of 4,184 J/(kg*°C) and the initial temperature of the water is 25°C.
For this, we use the formula:
Q = m * c * ΔT
where Q is the amount of heat added, m is the mass of the substance, c is the specific heat of the substance, and ΔT is the change in temperature of the substance.
We want to solve for ΔT, so it becomes:
ΔT = Q / (m * c)
Putting in the given values, we get:
ΔT = 50000 J / (0.9 kg * 4.184 J/(kg*°C))
ΔT = 14.56 °C
This tells us that the temperature of the water increased by 14.56°C. To find the final temperature, we need to add this to the initial temperature of 25°C:
Final temperature = 25°C + 14.56°C = 39.56°C
Therefore, the final temperature of the water is approximately 39.56°C.
To know more about specific heat, click here:
https://brainly.com/question/21041726
#SPJ11
Select the correct location on the image.
Identify the apparatus on the table that is best suited for a chemist to measure the volume of a liquid.

Answers
Answer:
The third one from the left–the graduated cylinder.
Explanation:
The laboratory apparatus that gives an "accurate" or "precise" measurement of a liquid's volume is the graduated cylinder. All you have to do is to pour the liquid into the cylinder and read its measurement using the calibrated scale.
The graduated cylinder comes in different sizes, which means the scale divisions will depend on its size. When reading the measurement, it is important to take note to read at the bottom of the meniscus because it gives the most accurate volume.
Answer:
graduated cylinder
Explanation:
How many protons, neutrons, and electrons are there?

Answers
Answer:
proton-27
electron-25
neutron-35
Explanation:
Which is an example of a physical change?
gasoline combusting
cake baking
salt dissolving
iron rusting
Answers
Answer:
salt dissolving
Explanation:
Hope this helps!
What volume is used to calculate the volume of a solid object
Answers
Answer:
cubic measurements
Explanation:
i.e. cubic meter or cubic centimeter
The elemental mass percent composition of ascorbic acid (vitamin C ) is 40.92% C , 4.58% H , and 54.50% O . Determine the empirical formula of ascorbic acid.
Answers
The empirical formula of ascorbic acid is C6H8O6.
Empirical formula
To calculate the empirical mass of a compound from the mass percentage of each element, the value of the molar mass of each element is used, which in this case corresponds to:
\(MM_C= 12g/mol\\MM_H=1g/mol\\MM_O=16g/mol\)
From this, we consider that the compound has 100 grams, so the number of moles of each element will be equal to:
\(C = \frac{40.92}{12} = 3.41 \\H = \frac{4.58}{1}=4.58\\ O =\frac{54.50}{16}=3.41\)
Now, divide all the values found by the smallest value, to find the amount present in each element:
\(C = \frac{3.41}{3.41} = 1\\ H = \frac{4.58}{3.41} = 1.34\\O = \frac{3.41}{3.41} = 1\)
As you can see, the value of moles of hydrogen resulted in a decimal number, so it is necessary to multiply all values by a number in common until the three meet as integers:
\(C = 1 \times 6 = 6\\H = 1.34 \times 6 = 8\\O = 6 \times 6 = 6\)
So, the empirical formula of ascorbic acid is C6H8O6.
Learn more about empirical and molecular formula in: brainly.com/question/11588623
In general, which solvent should be used to wash the solid material collected from a filtration?
Answers
The same solvent as the liquid in the original mixture should be used to wash the solid material collected from a filtration.
The solid material which is collected from the filtration is washed by that solvent in which that solid material is dissolved. Generally methanol solvent is used to wash the solid material collected from a filtration.
The filtration is the process in which solid particles present in liquid or gaseous fluid are removed by using of a filter.This filter is only permits or allow only the fluid to pass though it and retain the solid particles.The solid particles left is known as filtrate.learn about filtration
https://brainly.com/question/14530470
#SPJ4
Which of the four fundamental forces are involved in chemical bonding?
Answers
Answer:
The electromagnetic force is an important force in the chemical and biological sciences, as it is responsible for molecular connections like ionic bonding and hydrogen bonding.
Anyone get this please help me, can you do it step by step please

Answers
Answer:
approx 17.1429 days rounded up = 18days
Explanation:
400÷70 =5.7143 × 3 = 17. or rounded up = 18days
What is the effect of increasing pressure on the equilibrium? N2 + 3H2 ⇔ 2NH3 a) Equilibrium shifts in forward direction. b) Equilibrium shifts in backward direction. c) No effect d) It does not depends on pressure.
Answers
Answer:
a) Equilibrium shifts in forward direction.
Explanation:
If pressure is increased, equilibrium shifts to the side with the fewer moles of gas.
There are 4 moles of gas in the reactants and 2 moles of gas in the products.
The equilibrium will shift in the forward direction towards the products.
Hope that helps.
Answer:
A
Explanation:
What is the mass of water released by the heating? Show your work or explain your reasoning.
Answers
Answer:
Dividing the mass of the water lost by the original mass of hydrate used is equal to the fraction of water in the compound. Multiplying this fraction by 100 gives the percent water in the hydrate.
Explanation:
The amount of water throughout the compound has been determined by dividing the mass of water wasted mostly by original quantity of hydrate used. The above fraction can be multiplied by 100 to get the hydrate's water content in percentages.
What is mass ?The proportion of matter that makes up an object is quantified by its mass. The kilogram, or kg, would be the fundamental SI unit of mass.
What is hydrate?Any substance that contains water through the form of H2O molecules is referred to as a hydrate. This water content by weight can vary, but it is typically fixed. The most well-known hydrates seem to be crystalline solids which decompose once the attached water is removed.
Therefore , The amount of water throughout the compound has been determined by dividing the mass of water wasted mostly by original quantity of hydrate used. The above fraction can be multiplied by 100 to get the hydrate's water content in percentages.
To know more about mass and hydrate.
https://brainly.com/question/11202174
#SPJ3
the production of iron and carbon dioxide from iron 3 oxide and carbon monoxide is an exothermic reaction. how many kiljoules of heat are produced when 3.40 mol fe2o3 reacts with an excess of co
Answers
the reaction releases 1,066.3 kJ of heat.
The balanced chemical equation for the reaction between Fe2O3 and CO to produce Fe and CO2 is:
Fe2O3 + 3CO → 2Fe + 3CO2
According to the equation, 1 mole of Fe2O3 reacts with 3 moles of CO to produce 2 moles of Fe and 3 moles of CO2.
Since there is an excess of CO, we can assume that all of the Fe2O3 will react completely. Therefore, the number of moles of CO needed can be calculated as:
3.40 mol Fe2O3 × (3 mol CO / 1 mol Fe2O3) = 10.2 mol CO
So, 10.2 moles of CO are needed to react completely with 3.40 moles of Fe2O3.
The heat released by the reaction can be calculated using the standard enthalpy of formation (ΔHf°) values for the compounds involved in the reaction. The ΔHf° values for Fe2O3, CO, Fe, and CO2 are -824.2 kJ/mol, -110.5 kJ/mol, 0 kJ/mol, and -393.5 kJ/mol, respectively.
To calculate the heat released, we can use the following formula:
ΔH = ΣnΔHf°(products) - ΣnΔHf°(reactants)
where ΣnΔHf° is the sum of the standard enthalpies of formation for the products and reactants, and n is the stoichiometric coefficient.
Plugging in the values, we get:
ΔH = (2 mol × -393.5 kJ/mol) + (3 mol × 0 kJ/mol) - (1 mol × -824.2 kJ/mol) - (3 mol × -110.5 kJ/mol)
= -1,066.3 kJ
Therefore, the reaction releases 1,066.3 kJ of heat.
To know more about heat go through:-
https://brainly.com/question/934320
#SPJ4
what pressure, in kpa, would be equal to 803 mmhg?
Answers
803 mmHg is equal to 106.44 kPa. This can be calculated using the equation P (kPa) = P (mmHg) × 0.133322.
The pressure of 803 mmHg is equal to 106.44 kPa. This can be calculated by using the following equation: P (kPa) = P (mmHg) × 0.133322. Therefore, 803 mmHg is equal to 106.44 kPa. To understand this relationship better, pressure can be thought of as a measure of how much force is applied to a certain area. The unit of measure for pressure is the Pascal (Pa), and one kPa is equal to 1000 Pa. The unit of measure for pressure in mmHg is the millimeter of mercury (mmHg). One mmHg is equal to 133.322 Pa. Therefore, one kPa is equal to 7.5 mmHg. To convert from mmHg to kPa, the equation is
P (kPa) = P (mmHg) × 0.133322. Using this equation, the pressure of 803 mmHg can be converted to 106.44 kPa.
To learn more about kPa :
https://brainly.com/question/26843253
#SPJ11
ASAP MULTIPLE CHOICE
Who discovered the electron?
J.J. Thomson
John Dalton
Antoine Lavoisier
Robert Millikan