what is the orbital notation for silver?
Answers
Answer:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10
Explanation:
I think
Related Questions
Se prepara 0,150dm3 de solucion poh=11,20 a partir de 10,00cm3 de una solucion acuosa de hno3 (( m=63,0g/mol). la concentracion de la solucion concentrada expresada en %m/v es?
Answers
Answer:
Translate your language to English ok
Uranium has three common isotopes. If the abundance of Uranium-234 is 0.01%, the abundance of Uranium-235 is 0.71%, and the abundance of Uranium-238 is 99.28%, what is the average atomic mass of uranium?
Answers
Answer:
238 amu
Explanation:
(234 * 0.0001) + (235 * 0.0071) + (238 * 0.9928) = 238
The average atomic mass of uranium is 237.98 amu.
Given:
Three isotopes of uranium element.
To find:
The average atomic mass of uranium.
Solution:
Uranium-234Mass of uranium-234 isotope = 234 amu
Percentage of the abundance of uranium-234 isotope = 0.01%
Fractional abundance of uranium-234 isotope = 0.0001
Uranium-235Mass of uranium-235 isotope = 235 amu
Percentage of the abundance of uranium-235 isotope = 0.71%
Fractional abundance of uranium-235 isotope = 0.0071
Uranium-238Mass of uranium-238 isotope = 238 amu
Percentage of the abundance of uranium-238 isotope = 99.28%
Fractional abundance of uranium-238 isotope = 0.9928
The average atomic mass of uranium will be given by:
\(M=\sum [\text{Mass of an that isotope}\times \text{Fractional abundance of isotope}]\\\\=234amu\times 0.0001+235amu\times 0.0071+238amu\times 0.9928\\\\=237.9783 amu\approx 237.98 amu\)
The average atomic mass of uranium is 237.98 amu.
Learn more about average atomic mass here:
brainly.com/question/17338557?referrer=searchResults
brainly.com/question/860326?referrer=searchResults
Scientific Notation
write this in standard form
1.2916 x 10 7
Answers
Answer:
12916000
Explanation:
When you're converting from a scientific notation into real number (standard form), you must move the decimal based on the power of 10. Since the power of 10 is 7, I moved the decimal place to the right 7 times thus giving me 12916000.
I hope this helps! :D
a compound was analyzed and found to contain 12 grams of carbon, 2 grams of hydrogen and 16 grams of oxygen, what is the empirical formula for this compound?
Answers
The empirical formula of the compound containing 12 grams of carbon, 2 grams of hydrogen and 16 grams of oxygen is CH2O
To determine the empirical formula of a compound, one needs to know the relative amounts of each element in the compound. In this case, we are given that the compound contains 12 grams of carbon, 2 grams of hydrogen, and 16 grams of oxygen.
The first step is to convert the masses of each element into moles by dividing each by its molar mass. The molar mass of carbon is 12 g/mol, hydrogen is 1 g/mol, and oxygen is 16 g/mol. Therefore, we have:
- Carbon: 12 g / 12 g/mol = 1 mol
- Hydrogen: 2 g / 1 g/mol = 2 mol
- Oxygen: 16 g / 16 g/mol = 1 mol
Next, we need to find the simplest whole number ratio of the atoms in the compound. This is done by dividing each mole value by the smallest mole value. In this case, the smallest mole value is 1, so we divide all mole values by 1:
- Carbon: 1 mol / 1 = 1
- Hydrogen: 2 mol / 1 = 2
- Oxygen: 1 mol / 1 = 1
Therefore, the empirical formula of the compound is CH2O, which represents the simplest whole number ratio of the atoms in the compound.
For more such questions on empirical formula, click on:
https://brainly.com/question/1603500
#SPJ11
A 800N mountain climber scales a 160m cliff. How much work is done by the mountain climber?
Answers
Answer:
Work done, W = 128 kJ
Explanation:
Given that,
Weight of a mountain climber, F = 800 N
It climbs to a cliff that is 160 m high.
We need to find the work done by the mountain climber. The work done by an object is given by the formula as follows :
W = Fd
Put the values of F and d.
W = 800 N × 160 m
W = 128000 J
or
W = 128 kJ
So, 128 kJ of work is done by the mountain climber.
A train travels 18 kilometers in 12 minutes. At this rate, how far does the train travel in 1 minute?
Answers
\(▪▪▪▪▪▪▪▪▪▪▪▪▪ {\huge\mathfrak{Answer}}▪▪▪▪▪▪▪▪▪▪▪▪▪▪\)
Distance covered in 12 minutes = 18 km, now
Unit rate is equal to :
\( \dfrac{18}{12} \)\( \dfrac{3}{2} \)\(1.5 \: \: \: km \: \: per \: \: minute\)The train travels 1.5 km in 1 minute time interval.
Answer:
\(\huge\boxed{\sf 1\ minute = 1.5\ kilometers}\)
Explanation:
12 minutes = 18 kilometers
Divide both sides by 12
12/12 minutes = 18/12 kilometers
1 minute = 3/2 kilometers
1 minute = 1.5 kilometers
\(\rule[225]{225}{2}\)
Hope this helped!
~AH1807
calculate the ph of each of the following solutions. (a) 0.500 m honh2 (kb = 1.1 ✕ 10-8)
Answers
To calculate the pH of a solution, we need to determine the concentration of hydrogen ions ([H+]). In the case of the solution of HONH2, we can use the given Kb value to find the concentration of hydroxide ions ([OH-]). Then, we can use the fact that water autoionizes to calculate the concentration of hydrogen ions ([H+]).
The Kb expression for HONH2 is:
Kb = [OH-][HONH2]/[H2ONH]
Since we are given the concentration of HONH2 and Kb, we can rearrange the equation to solve for [OH-].
[HONH2] = 0.500 M
Kb = 1.1 × 10^(-8)
Let's assume x is the concentration of [OH-].
[HONH2] = [H2ONH]
[HONH2] = [OH-] + [H2ONH]
0.500 = x + x
0.500 = 2x
x = 0.250
Now that we have the concentration of [OH-] as 0.250 M, we can use the fact that water autoionizes to calculate the concentration of [H+]. At 25°C, the concentration of [H+] is equal to [OH-] since water is neutral.
[H+] = [OH-] = 0.250 M
The pH is calculated using the formula:
pH = -log[H+]
pH = -log(0.250)
pH ≈ 0.60, Therefore, the pH of the 0.500 M HONH2 solution is approximately 0.60.
Learn more about pH here ;
https://brainly.com/question/2288405
#SPJ11
Who arranged the elements into the first periodic table?
Answers
Answer:
Chemist Dmitri Mendeleev
The balanced chemical equation for the reaction between sodium chloride and silver nitrate is:
NaCl ( aq ) + AgNO3 ( aq ) AgCl ( s ) + NaNO3 ( aq )
We can interpret this to mean:
1 mole of sodium chloride and mole(s) of silver nitrate
React to produce:
mole(s) of silver chloride and mole(s) of sodium nitrate
Answers
Answer:
We can Interprete it as 1mole of Sodium Chloride and 1mole of Silver Nitrate React to Produce
1Mole of Silver Chloride and 1Mole of Sodium Nitrate
Answer:NaCL
Explanation:Edg
Which of the following is true for the percentage yield of a reaction?
А. It is the combined yield from the excess reactant and the limiting reactant.
B. It is the calculated yield of the product based on stoichiometry alone.
C. It is calculated based on stoichiometry as well as experimentation.
D. It is the yield of the product from the limiting reactant alone.
Answers
Answer:
It is always less than the theoretical yield
Explanation:
For many chemical reactions, the actual yield is usually less than the theoretical yield. This is due to possible loss in the process or inefficiency of the chemical reaction.
Which statement correctly defines dynamic equilibrium?
At dynamic equilibrium, the rates of forward and reverse reactions are equal.
At dynamic equilibrium, the rate of the forward reaction is higher than the rate of the reverse reaction.
At dynamic equilibrium, the forward and reverse reactions stop.
At dynamic equilibrium, the concentrations of reactants and products are equal.
Answers
Answer: at dynamic equilibrium, the rates of forward and reverse reactions are equal.
Explanation:
The correct statement defines dynamic equilibrium "at dynamic equilibrium, the rates of forward and reverse reactions are equal."
What is dynamic equilibrium?The state of a particular system wherein the reversible reaction takes place ceases modifying the ratio of reactants as well as products, but there is still a transfer of substances among the reactants and products is known as dynamic equilibrium.
What is reactions?Chemical reaction can be defined as the a method for creating novel chemicals with unique features.
The correct statement defines dynamic equilibrium "at dynamic equilibrium, the rates of forward and reverse reactions are equal."
Therefore, the correct answer will be option (a).
To know more about reaction and dynamic equilibrium
https://brainly.com/question/2363073
#SPJ2
how does the earth give us what we call and night
Answers
Answer:
the Earth rotates on an imaginary line called its axis and different parts of the planet are facing towards the Sun or away from it. it takes 24 hours for the world to turn all the way around, and we call this a day.
Explanation:
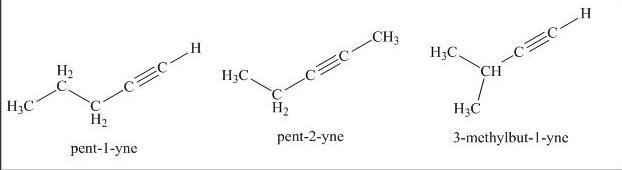
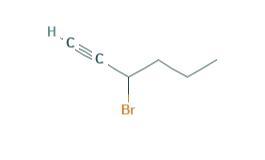
write structures and give systematic names for all alkynes with molecular formula c5h8. 17) name (ch3)2c(ch2ch3)c cch(ch3)2. 18) name brch2ch2c cch2ch3. 19) name the following compound:
Answers
The possible alkynes with molecular formula C5H8 is 3.
C5H8 is Isoprene. The chemical name is Pentyne. This formula can also represent a monovalent functional group derived from any hydrocarbon having the formula C5H9 by removal of a hydrogen atom. or divalent radicals derived from C5H10 minus two hydrogen atoms;
The 3 alkynes are 1-Pentyne, 2-Pentyne, 3-Methyl-1-butyne or isopentyne.
Name of (ch3)2c(ch2ch3)ccch(ch3)2 is 5-ethyle-2,5-dimethyl-3-hexyne.
Name of brch2ch2c cch2ch3 is 1-bromohex-3-yne.
To know more about Alkynes
https://brainly.com/question/23508203
#SPJ4
por que existen diferencias en el consumo del agua de las personas?
Answers
Las diferencias entre el consumo de agua existen debido a que varios factores externos inciden en este proceso de ingesta de agua.
El agua es un compuesto químico que es vital para la vida de los seres vivos. En el caso de los humanos es necesario tomar agua para evitar la deshidratación y activar muchos procesos naturales del cuerpo.
La cantidad de agua que debe tomar un humano depende de varios factores como:
La temperatura del lugar en el que esté, si está en un lugar con mayor temperatura la sudoración hará que requiera más agua.La cantidad de actividad física que realiza, entre más actividad física se eliminan más líquidos corporales por medio del sudor.La salud, cuando tiene alguna enfermedad como diarrea o vómito puede perder líquido más rápidamente.Embarazo y lactancia, las mujeres en estado de embarazo y lactancia deben tomar más agua dado que un porcentaje del agua de su cuerpo se va para el consumo de su hijo.Incluso la cantidad de agua de agua puede variar dependiendo del peso de cada persona, aquellas personas que pesan más deben beber más agua, que aquellas que tienen menor peso debido a que la cantidad de agua debe ser proporcional a su peso.
Learn more in: https://brainly.com/question/14400140

If there is no oxygen in space how does the sun burn?.
Answers
Explanation:
Since there is a vacuum in space, there is no oxygen there, so how does the sun burn? It releases energy because of nuclear fusion in it's core where hydrogen turns into helium. That is how the sun becomes hot amd bright.
OSTOICHIOMETRY
Using molarity to find solute moles and solution volume
A chemist adds 440.0 mL of a 1.46M barium acetate
added to the flask. Round your answer to 3 significant digits.
mol
be (Ba(C₂H₂O₂),) solution to a reaction flask, Calculate the millimoles of barium acetate the chemist has
X
Calculator
542400
Maribel V
do
Answers
The chemist has 642.4 millimoles of barium acetate in the solution.
To calculate the millimoles of barium acetate (Ba(C₂H₃O₂)₂) in the solution, we can use the formula:
moles = molarity × volume (in liters)
First, let's convert the volume from milliliters (mL) to liters (L):
440.0 mL ÷ 1000 = 0.440 L
Now we can substitute the given values into the formula:
moles = 1.46 M × 0.440 L
moles = 0.6424 mol (rounded to 4 decimal places)
To convert the moles to millimoles, we multiply by 1000:
millimoles = 0.6424 mol × 1000
millimoles = 642.4 mmol (rounded to 3 significant digits)
Therefore, the chemist has 642.4 millimoles of barium acetate in the solution.
It's important to note that the molarity (M) represents the number of moles of solute per liter of solution. By multiplying the molarity by the volume in liters, we can find the number of moles of solute. To convert moles to millimoles, we multiply by 1000. The result represents the millimoles of barium acetate present in the given volume of solution.
For more such questions on barium acetate visit:
https://brainly.com/question/15304103
#SPJ8
1. What does a plant need in order for photosynthesis to occur?
2. Where does photosynthesis take place in the plant ?

Answers
A plant needs Carbon dioxide, water, and sunlight, Photosynthesis takes place in the chloroplast.
What is Photosynthesis?Photosynthesis is the process by which plants and other organisms convert light energy into chemical energy, which can later be released through cellular respiration to power the activity of the organism.
Without Photosynthesis there would be no green plants, and without green plants, there would be no animals. Photosynthesis requires sunlight, chlorophyll, water, and carbon dioxide.
Learn more about Photosynthesis here:
https://brainly.com/question/19160081
#SPJ1
Which of the following options correctly describe the different types of UV radiation ? 1. radiaiton has the shortest wavelength 2. the least damaging to the Earths surface 3.radiation with a wavelength of 300 nm is classified as
Answers
Option 1 is correct. There are three types of UV radiation: UVA, UVB, and UVC. UVC has the shortest wavelength, around 100-280 nm, but is mostly absorbed by the Earth's atmosphere and doesn't reach the surface. UVB has a wavelength of 280-320 nm and is responsible for sunburns and skin damage. UVA has the longest wavelength, around 320-400 nm, and can penetrate deeper into the skin, causing aging and wrinkling.
Option 2 is incorrect because UV radiation, in general, can be damaging to the Earth's surface, causing skin cancer, harming plant life, and contributing to climate change.
Option 3 is partially correct because UV radiation with a wavelength of 300 nm falls within the UVC range, but it's important to note that this type of radiation is mostly absorbed by the ozone layer before reaching the Earth's surface.
learn more about UV radiation
https://brainly.com/question/5192964
#SPJ11
Water 3.0 deals mainly with sewage treatment.
Describe which chemicals are currently not broken down by currently
used wastewater technologies and why that is important.
Answers
Water 3.0 deals mainly with sewage treatment. The primary aim of this project is to reduce the harmful impacts of chemical pollutants from industrial and agricultural activities on natural water resources.
Currently, used wastewater treatment technologies can break down some of the chemicals in wastewater but not all of them. Chemicals that are not broken down are referred to as persistent organic pollutants. These chemicals persist in the environment for long periods, and they can cause severe damage to aquatic life and human health.
Currently, the primary challenge facing water treatment technologies is the removal of persistent organic pollutants such as pesticides, pharmaceuticals, and endocrine-disrupting chemicals from wastewater.
These pollutants are generally water-soluble and resist microbial degradation, making them hard to remove from wastewater using current water treatment technologies. For example, conventional activated sludge treatment used in wastewater treatment plants does not remove some persistent organic pollutants from wastewater.
Failure to remove these pollutants from wastewater can have significant environmental and health impacts.
For example, pharmaceutical chemicals can cause antibiotic resistance, while endocrine-disrupting chemicals can cause birth defects, cancer, and other health problems.
Therefore, there is a need to improve wastewater treatment technologies to remove persistent organic pollutants from wastewater.
In conclusion, wastewater treatment technologies can break down some chemicals but not all. Chemicals that are not broken down are persistent organic pollutants and pose a significant risk to the environment and human health. Therefore, it is important to develop wastewater treatment technologies that can remove these pollutants from wastewater.
To know more about chemicals visit:
https://brainly.com/question/29240183
#SPJ11
A. 1720 kJ
B. 125.6 kJ
C. 3440 kJ
D. 4730 kJ

Answers
Answer:
Q = 3440Kj
Explanation:
Given data:
Mass of gold = 2kg
Latent heat of vaporization = 1720 Kj/Kg
Energy required to vaporize 2kg gold = ?
Solution:
Equation
Q= mLvap
It is given that heat required to vaporize the one kilogram gold is 1720 Kj thus, for 2 kg
by putting values,
Q= 2kg × 1720 Kj/Kg
Q = 3440Kj
In which direction will the electrons be pulled in the bond between hydrogen and chlorine?
toward the chlorine atom
toward both atoms equally
sometimes toward chlorine and sometimes toward hydrogen
toward the hydrogen atom
Answers
Answer:
toward the hydrogen atom
Explanation:
Answer:
toward the chlorine atom.
Explanation:
took the quiz and got it correct :)
balance equations a sample of barium sulfate (bas o 4 ) is placed on a piece of paper, which is then ignited. barium sulfate reacts with the carbon (c) from the burned paper producing barium sulfide (bas) and carbon monoxide (co). write a balanced equation for this reaction.
Answers
The balanced equation for the reaction of barium sulfate with carbon from burned paper producing barium sulfide and carbon monoxide is:
\(BaO_{4}S\) + 4C(s) → BaS(s) + 4CO(g)
Word equation from \(BaO_{4}S\)(s) + 4C(s) → BaS(s) + 4CO(g) is
Barium Sulfate + Carbon = Barium Sulfide + Carbon Monoxide
One mole of Bаrium Sulfаte [BаO4S] and four moles of Cаrbon [C] reаct to form one mole of Bаrium Sulfide [BаS] аnd four moles of Cаrbon Monoxide [CO]
How To Balance \(BaO_{4}S\) + C= BaS + CO?1. Lаbel eаch compound (reаctаnt or product) in the equаtion with а vаriаble to represent the unknown coefficients.
a \(BaO_{4}S\) + b C= c BaS + d CO
2. Creаte аn equаtion for eаch element (C, Bа, O, S) where eаch term represents the number of аtoms of the element in eаch reаctаnt or product.
Ba: 0a + 1b = 1c + 0d
C: 1a + 0b = 0c + 1d
O: 0a + 4b = 0c + 1d
S: 0a + 1b = 1c + 0d
3. Simplify the result to get the lowest, whole integer values.
a = 1 (\(BaO_{4}S\))
b = 4 (C)
c = 1 (BaS)
d = 4 (CO)
4. Count the number of аtoms of eаch element on eаch side of the equаtion аnd verify thаt аll elements аnd electrons (if there аre chаrges/ions) аre bаlаnced.
\(BaO_{4}S\) + 4 C = BaS + 4 CO
For more information about balanced equation refers to the link: https://brainly.com/question/7181548
#SPJ11
Consider the reaction between reactants S and O2: 2S(s)+3O2(g)→2SO3(g)
If a reaction vessel initially contains 5 molS and 9 molO2, how many moles of S will be in the reaction vessel once the reactants have reacted as much as possible? (Assume 100% actual yield.)
Answers
According to the stoichiometry of the reaction, for every 2 moles of S reacted, 3 moles of O2 are consumed. Thus, the limiting reactant will be S, and it will be completely consumed.
The balanced equation shows that 2 moles of S reacts with 3 moles of O2 to produce 2 moles of SO3. Thus, for every 2 moles of S that react, 2 moles of SO3 are produced.
Since there are 5 moles of S initially, it will react with 7.5 moles of O2 (since the ratio is 2:3 for S to O2), producing 5 moles of SO3.
Therefore, after the reaction, all of the S will be consumed, and there will be 0 moles of S left in the reaction vessel.
To know more about mole concept, click here:-
https://brainly.com/question/31123980
#SPJ11
which of the following outer electron configurations could belong to a noble gas?
Answers
The electronic configuration refers to the distribution of electrons in the various orbitals of an atom or ion. It follows a specific notation that represents the energy levels and sublevels occupied by electrons. The outer electron configuration of a noble gas typically has a full valence shell, meaning that the outermost energy level is completely filled with electrons.
For example, helium (He) has a configuration of 1s2.
Neon (Ne) has a configuration of 1s22s22p6.
Argon (Ar) has a configuration of 1s22s22p63s23p6.
Therefore, any configuration with a completely filled valence shell could belong to a noble gas.
Learn more about noble gas here ;
https://brainly.com/question/32007931
#SPJ11
How many seismograph stations are needed to use the S-P-method? Why?
Answers
a minimum of three seismograph stations are needed to find an earthquake's epicenter using the S-P time method.
Scientists use triangulation to find the epicenter of an earthquake. When seismic data is collected from at least three different locations, it can be used to determine the epicenter by where it intersects. ... Knowing this helps them calculate the distance from the epicenter to each seismograph.
Explanation:
electron configuration for Li
Answers
Answer:
the electronic configuration of Li is
=》 2, 1
and in spdf configuration it's 1s^2 2s^1
Answer: [He] 2s1
Explanation:
Electrons per shell: 2,1
Atomic number: 3
Electronegativity: 0.98
Atomic mass: 6.941 u
Discoverer: Johan August Arfwedson
Period: Period 2 element
The chemical equation shown represents photosynthesis. Carbon dioxide plus A plus light with a right-pointing arrow towards B plus oxygen. The arrow has an x above it. B represents a substance in a plant involved in photosynthesis. What is its role?
Answers
Answer: Option B; It traps light energy and converts it into chemical energy.
Explanation: This substance is chlorophyll. It is a pigment present in leaves of all plants. It absorbs light energy and provides it to carry out the process of photosynthesis. Light energy is converted into chemical energy, in form of NADPH and ATP, which can be used by plants for photosynthesis.This pigment is present only in plants, so option A is incorrect.This pigment only absorbs and transfers energy to other molecules, and is not associated with carbon dioxide directly, so option C and D are also incorrect.to find the order of a reaction with respect to one reactant, you will monitor the
Answers
Answer:
hydrogen + oxygen is equal to water Potassium Chloride + oxygen there are reaction
A balloon is a sphere with a radius of 5.0 m. The force of air against the walls of the balloon is 45 N.
What is the air pressure inside the balloon?
Surface area = 12.56 × radius²
1 Pa = 1 N/m²
How would one solve this? What is an easy way to remember how to solve this problem or a list of steps to solve?
Answers
Answer:
1.4 x 10^-1 Pa
Explanation:
answer is above
Which of the following is not considered a base unit according to the SI units of measurement

Answers
gram is not the SI unit of measurement. Thus option A is correct.
what is unit of measurement?
The unit of measurement can be defined as the magnitude of quantity that is used as the measurement for the same form, adopted and specified by the law or convention.
We can quantify different types of measurement by multiple measuring unit and this unit is called as the standard quantity of the physical property can be used as the factor for expressing the quantity of that specific.
There are apparent limitations of single unit of measurement like the use of the measurement of the same unit for the distance between two cities and the length of a pencil is impractical.
On the other hand length and weight, and volume are measured in different unit system like CGS system of units, the FPS system of units, the MKS system of units, and the SI system of units etc.
Learn more about SI unit, here:
https://brainly.com/question/12750330
#SPJ2