Answers
Answer:
2 - Butyne
Explanation:
The name of the molecule with a carbon atoms arranged in a straight chain with a triple bond between the second and third carbons is 2 - Butyne.
2- Butyne is an alkyne with structural formula given below. Some of the properties of Butyne include it is a produced artificially, it is volatile and colorless in nature.
Hence, the given molecules described is 2 - Butyne.

Related Questions
which of the following has the largest radius k,Na+,Na,k+
Answers
Answer: it's k
Explanation:
Same reason as the person above me
The order of the alternatives from largest to lowest atomic radius is K > Na > \(\rm Na^+\) > \(\rm K^+\).
The atomic radius usually decreases from left to right across the periodic table due to increasing effective nuclear charge, which attracts electrons more strongly and reduces the atomic radius. As a result, among the options offered K (potassium) has the largest atomic radius. Because the atomic radius shrinks from left to right, K (potassium) has a larger atomic radius than Na (sodium).
In addition, when an atom loses an electron to become an ion, its radius decreases. As a result, the \(\rm Na^+\) ion has a smaller radius than Na. The radius of potassium ion is greater than that of sodium ion because potassium is in the same period as sodium but is one group to the left, resulting in a larger atomic radius.
To know more about radius, here:
https://brainly.com/question/24051825
#SPJ4
What amount of heat energy would be necessary to raise the temperature of 100 g of water at room temperature (25°C) to the boiling point (100°C)? The specific heat of water is 1.0 cal/g°C.
75 kcal
100 kcal
750 kcal
7.5 kcal
Answers
Answer:
7.5 kcal
Explanation:
1.0 cal /g-C * 100 g * (100- 25 C) = 7500 cal = 7.5 kcal
The amount of heat energy necessary to raise the temperature of 100 g of water at room temperature (25°C) to the boiling point (100°C) is 7.5 kcal.
Given to us the mass of water, the specific heat of water, and the change in temperature, we need to calculate the amount of heat energy.
m = 100 g
c = 1.0 cal/g°C
ΔT = (100 °C - 25 °C) = 75 °C
To calculate the amount of heat energy required, we can use the formula:
Q = m × c× ΔT
Where:
Q = heat energy (in calories)
m = mass of water (in grams)
c = specific heat of water (in cal/g°C)
ΔT = change in temperature (in °C)
Substituting the values into the formula:
Q = 100 g × 1.0 cal/g°C × 75 °C
Q = 7500 cal
7500 cal = 7.5 kcal
Therefore, the amount of heat energy required to raise the temperature of 100 g of water from 25 °C to 100 °C is 7500 calories, which is equivalent to 7.5 kcal.
Learn more about specific heat here:
https://brainly.com/question/31608647
#SPJ2
How many kilojoules of heat are needed to raise the temperature of 10g of aluminum from 22 degrees C to 55 degrees C, if the specific heat of aluminum is .901 j/gc?
Answers
Answer:
name four agricultural inputs are subsidized by the government
0.297 kJ of heat is needed to raise the temperature of 10g of aluminum from 22 degrees Celsius to 55 degrees Celsius.
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
It is a measure of how much energy it takes to raise the temperature of a substance. It is the amount of heat necessary to raise one mass unit of that substance by one temperature unit.
It is given by the formula -
Q = mcΔT
where, Q = amount of heat
m = mass
c = specific heat
ΔT = Change in temperature
Given,
mass = 10g
c = 0.901J/g⁰C
Initial temperature (T₁) = 22⁰C
Final Temperature (T₂) = 55⁰C
Q = mcΔT
= 10 × 0.901 × (55 -22)
= 297.33 J = 0.297 kJ
Learn more about Specific heat, here:
https://brainly.com/question/31608647
#SPJ1
Calculate the volume of chlorine molecules produced at room temperature and pressure, when 234g of sodium chloride are electrolysed. (1 mole of chlorine molecules has a volume of 24 dm³ at room temperature and pressure).
Answers
The volume of chlorine molecules produced at STP would be 96 dm³.
Stoichiometric problemSodium chloride ionizes during electrolysis to produce sodium and chlorine ions as follows:
\(NaCl --- > Na^+ + Cl^-\)
This means that 1 mole of sodium chloride will produce 1 mole of sodium ion and 1 mole of chlorine ion respectively.
Recall that: mole = mass/molar mass
Hence, 234 g of sodium chloride will give:
234/58.44 = 4.00 moles.
Thus, the equivalent number of moles of chlorine produced by 234 g of sodium chloride will be 4 moles.
Recall that:
1 mole of every gas at Standard Temperature and Pressure = 24 Liters.
Hence:
4 moles of chlorine = 4 x 24 = 96 Liters or 96 dm³.
More on stoichiometric problems can be found here: https://brainly.com/question/14465605
#SPJ1
How are ocean waves formed? gravity energy transfer conduction radiation

Answers
Answer:
I apologize that I'm late and all But your answer is B. Energy Transfer.
Explanation:
The waves transfer energy to the sand for example.
2. How can you change the concentration of a solution, yet keep the number of moles of solute the same
Answers
One can change the concentration of a solution while keeping the number of moles of solute the same by reducing the volume of solvent in the solution.
What is molarity?The molarity of a solution is the number of moles of solute per unit volume of solvent.
The number of moles of solute in a unit volume of solvent can be kept constant while the volume of the solvent reduced in order to increase the concentration of a solution.
For example, a solution with 2 moles of solute in 1 L of solvent will have a molarity of 2 M.
If the amount of solvent is reduced to 0.5 L, the molarity will become:
2/0.5 = 4 M
More on molarity can be found here: https://brainly.com/question/12127540
If we have 1.23 mol of NaOH in solution and 0.85 mol of Cl2 gas is available to react, which one is the limiting reactant? Give your reason.
Answers
Answer:
NaOH is the limiting reactant.
Explanation:
Hello there!
In this case, since the reaction taking place between sodium hydroxide and chlorine has is:
\(NaOH+Cl_2\rightarrow NaCl+NaClO+H_2O\)
Which must be balanced according to the law of conservation of mass:
\(2NaOH+Cl_2\rightarrow NaCl+NaClO+H_2O\)
Whereas there is a 2:1 mole ratio of NaOH to Cl2, which means that the moles of the former that are consumed by 0.85 moles of the latter are:
\(n_{NaOH}=0.85molCl_2*\frac{2molNaOH}{1molCl_2}\\\\n_{ NaOH}=1.7molNaOH\)
Therefore, since we just have 1.23 moles out of 1.70 moles of NaOH, we infer this is the limiting reactant.
Regards!
Aqueous aluminum bromide and chlorine gas react to form aqueous
aluminum
chloride and bromine gas
in a equation
Answers
Answer:
2AlBr₃\(_{aq}\) + 3Cl₂\(_{g}\) → 2AlCl₃\(_{aq}\) + 3Br₂\(_{g}\)
Explanation:
The word equation is:
Aqueous aluminum bromide and chlorine gas react to form aqueous aluminum chloride and bromine gas
Reactants:
aluminum bromide = AlBr₃
chlorine gas = Cl₂
Products:
aluminum chloride = AlCl₃
bromine gas = Br₂
So;
2AlBr₃\(_{aq}\) + 3Cl₂\(_{g}\) → 2AlCl₃\(_{aq}\) + 3Br₂\(_{g}\)
One of the products of the combustion reaction below:
C5H12 + O2 → CO2 + _____ is
1. C2H5OH 3. CH4
2. H2O 4. NO
Answers
Answer:
H₂O
Explanation:
Chemical equation:
C₅H₁₂ + O₂ → CO₂ + H₂O
Balanced chemical equation:
C₅H₁₂ + 8O₂ → 5CO₂ + 6H₂O
The given reaction is combustion reaction. The balance equation shows that there are equal number of moles of carbon, hydrogen and oxygen on both side of equation thus it follows the law of conservation of mass.
Combustion reaction:
The reaction in which substance react with oxygen and produced carbon dioxide and water.
Mostly hydrocarbons burns in the presence of oxygen and form carbon dioxide and water.
The radius of a silver atom is 145 pm. How many silver atoms would have to be laid side by side to span a distance of 2.31
mm?
atoms
M
Answers
Given that the radius of a silver atom is 145 pm, it would take \(7.97 \times 10^{6}\) silver atoms laid side by side to span a distance of 2.31 mm.
The radius of a silver atom is 145 pm. Then, the diameter (twice the radius) is:
\(d = 2 \times 145 pm = 290 pm\)
We will convert 290 pm to millimeters. We will use the conversion factors:
1 m = 10¹² pm1 m = 10³ mm\(290 pm \times \frac{1m}{10^{12}pm } \times \frac{10^{3}mm }{1m} = 2.90 \times 10^{-7} mm\)
If the diameter of 1 silver atom is 2.90 × 10⁻⁷ mm, the number of silver atoms required to span a distance of 2.31 mm is:
\(2.31 mm \times \frac{1Ag\ atom}{2.90 \times 10^{-7}mm } = 7.97 \times 10^{6} Ag\ atom\)
\(7.97 \times 10^{6}\) silver atoms span a distance of 2.31 mm.
You can learn more about unit conversions here: brainly.com/question/19420601
what is the molarity of a solutoin which contains 38.5 g of sodium chloride disolved in 325 ml solution ?
Answers
Answer:
Molarity = 2.02 M
Explanation:
Given data:
Molarity of solution = ?
Mass of sodium chloride = 38.5 g
Volume of solution = 325 mL (0.325 L)
Solution:
Number of moles = mass/molar mass
Number of moles = 38.5 g/ 58.44 g/mol
Number of moles = 0.658 mol
Molarity:
Molarity = number of moles / volume of solution in L
Molarity = 0.658 mol /0.325 L
Molarity = 2.02 M
convert 9.3 x 10^15 atoms of lead to moles of lead.
Answers
Answer:
9.3×1015 atoms Pb make up 1.5×10−8mol Pb
A tank of bromine gas is initially at a pressure of 5.6 atm, a temperature of 67°C, and has a volume of 97 L. The tank is expanded to 100 L and has a new pressure of 8 atm.
What is the new temperature of the gas in Kelvin?
Answers
The new temperature of the bromine gas as the tank is expanded to the given volume and pressure is 500.96 Kelvin.
Combined gas lawCombined gas law put together both Boyle's Law, Charles's Law, and Gay-Lussac's Law. It states that "the ratio of the product of volume and pressure and the absolute temperature of a gas is equal to a constant.
It is expressed as;
P₁V₁/T₁ = P₂V₂/T₂
Given the data in the question;
Initial volume V₁ = 97LInitial pressure P₁ = 5.6atmInitial temperature T₁ = 67°C = 340.15KFinal volume V₂ = 100LFinal pressure P₂ = 8.0atmFinal temperature T₂ = ?To calculate the new temperature the gas, we subtsitute our given values into the expression above.
P₁V₁/T₁ = P₂V₂/T₂
P₁V₁T₂ = P₂V₂T₁
T₂ = P₂V₂T₁/P₁V₁
T₂ = ( 8.0atm × 100L × 340.15K ) / ( 5.6atm × 97L )
T₂ = 272120LatmK / 543.2Latm
T₂ = 500.96K
Therefore, the new temperature of the bromine gas as the tank is expanded to the given volume and pressure is 500.96 Kelvin.
Learn more about the combined gas law here: brainly.com/question/25944795
What is the physical makeup of water?
Answers
Water is composed of two hydrogen atoms and one oxygen atom
i. red the answers to this chemistry quiz sheet please!

Answers
1. decreases.
According to Boyle's Law, the relation between pressure and volume cab be stated as PV = k, where P is the pressure inside the container and V is the volume of the container.
So, pressure and volume are inversely proportional. If volume decreases pressure in the container increases and vice-versa. But this law is obeyed until the temperature and amount of gas remain unchanged.
2. Solids
Only solids are the substances which have fixed volume, changing shape and are not compressible.
3. 22.4 liters
At STP (Standard Temperature and Pressure), one mole of any ideal gas occupies a volume of approximately 22.4 liters.
4. D.
According to the kinetic Molecular Theory, solid's molecules move very quickly with high kinetic energy.
5. direct
Temperature and volume have direct relationship according to ideal gas law equation PV = nRT. We can see that temperature (T) and volume (V) are directly related.
6. Indirect
Pressure and volume have direct relationship according to ideal gas law equation PV = nRT. We can see that Pressure (T) and volume (V) are indirectly related.
7. P1 V1/n1 T1 = P2 V2/n2 T2
As the values V = 0.35 L, T = 18. degree celsius and P = 980 mmHg and constant R = 8.314 J/(mol·K), we can easily find V2 easily.
8. PV = nRT
The ideal gas law equation PV = nRT can be used fing V as the values for P, T, n are given in the problem and R value is a constant.
Imagine that you mix 25 g of water at 25 ºC with 25 g of water at 65 ºC. Predict the final temperature of the sample.
Answers
The final temperature of the mixture given that 25 g of water at 25 °C is mixed with 25 g of water at 65 °C, is 45 °C
How do i determine the final temperature of the mixture?The final temperature of the mixture can be obtained by calculating the equilibrium temperature of the mixture. This is shown below:
Mass of cold water (M) = 25 gTemperature of cold water (T) = 25 °CMass of warm water (Mᵥᵥ) = 25 gTemperature of warm water (Tᵥᵥ) = 65 °CEquilibrium temperature (Tₑ) =?Heat loss by warm water = Heat gain by cold water
MᵥᵥC(Tᵥᵥ - Tₑ) = MC(Tₑ - T)
Cancel out C
Mᵥᵥ(Tᵥᵥ - Tₑ) = M(Tₑ - T)
25× (65 - Tₑ) = 25 × (Tₑ - 25)
Cancel out 25
65 - Tₑ = Tₑ - 25
Collect like terms
65 + 25 = Tₑ + Tₑ
90 = 2Tₑ
Divide both side by 2
Tₑ = 90 / 2
Tₑ = 45 °C
Thus, we can conclude that the final temperature the mixture is 45 °C
Learn more about temperature:
https://brainly.com/question/14281142
#SPJ1
what do elemens in the same period have in common?
Answers
Answer:
There stable oxidation state is +3.
And they have only three energy levels
Convert 100.6 Kelvin to degrees C.
°C = K - 273
[?] °C
Answers
Answer:
-172.6 °C
Explanation:
You want to know the Celsius equivalent of the temperature 100.6 K.
ConversionThe relation is ...
C = K - 273.15
C = 100.6 -273.15 = -172.55
The temperature is -172.55 °C, about -172.6 °C.
__
Additional comment
We have rounded to tenths, because that is precision of the temperature given. If you use 273 as the conversion constant, you will get -172.4.
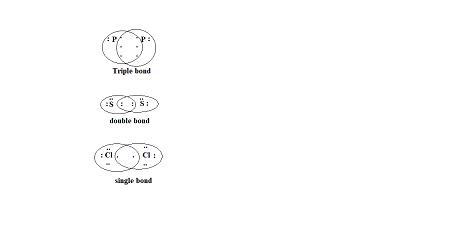
Rank the following series of molecules or ions in order of decreasing bond energy using their bond order to predict relative magnitude: chlorine: Cl2, sulfur: S2, phosphorus: P2.
Answers
Answer:
P2>S2>Cl2 is the order of bond energy of the given molecules.
Explanation:
The bonding in each molecule is shown below:
Thus, between each P-atom, there exists a triple bond.
Between two S-atoms there exists a double bond.
Between two chlorine atoms, there exists a single bond.
As the number of bonds increases between the given atoms, then bond energy required to break the bonds also increases.
Thus, the bond order is shown below:
\(P_2>S_2>Cl_2\).
A mouthwash contains 22.5% alcohol(p=0.9652). If the bottle of mouthwash contains 355 mL. How many milliliters of alcohol are present(p=0.7893)?
Answers
The volume of the alcohol can be obtained as 79.9 mL.
What is the volume of the alcohol?We know that there are many different unit of concentration. The unit of concentration that we are going to focus on here is the volume per volume percent. This has to do with the volume of solute in a given volume of the solvent.
Thus we have;
Volume solution = 355 mL
Volume percent of the alcohol = 22.5%
Actual volume of the alcohol = x
We now have;
Volume percent = Volume of the alcohol (x)/volume of the solution * 100/1
22.5 = x/355 * 100/1
22.5 = 100x/355
x = 22.5 * 355/100
x = 79.9 mL
Learn more about volume percent :https://brainly.com/question/15461083
#SPJ1
Missing parts;
A mouthwash contains 22.5% (v/v) alcohol. If the bottle of mouthwash contains 355 mL, what is the volume, in milliliters, of alcohol
At 25.0° C, a 10.00 L vessel is filled with 5.25 moles of Gas A and 7.75 moles of Gas B. What is the total pressure in atm?
Answers
Answer:
23.12 atm
Explanation:
First, add together the moles of the two samples:
5.25 moles + 4.20 moles = 9.45 moles
273 + 25 = 298 K for the temperature
volume is 10.0 L
Since we have moles now, we have to rearrange our ideal law equation to solve for pressure:
\(P = \frac{nRT}{V}\)
\(\frac{(9.45 moles) X (0.08206) X (298 K)}{10.0 L}\)
9.45 X .08206 X 298 all divided by 10.0 = 23.09202 atm (or 23.12)
Aniyah has a special type of paper that can permanently change color, she wonders whether light can cause the paper”s color to change. The table below shows what happens when different types of light hit the paper
Answers
The color of the paper may change as a result of the UV light.
Yes, that is conceivable if the table you are referring to shows that ultraviolet (UV) radiation can cause the paper to change colour.
Compared to visible light, UV light is more energetic and has shorter wavelengths. The molecules of the paper may experience a chemical reaction as a result of this high-energy radiation, changing the colour of the paper.
The term "photochemical reaction" or "photochemistry" refers to this process. The molecules of the paper are affected by UV radiation, which can result in chemical reactions that create new compounds with various colours.
For instance, some dyes have chemical connections that are UV-sensitive. These connections may rupture in the presence of UV radiation, changing the dye's colour.
learn more about ultraviolet light here
https://brainly.com/question/27778727
#SPJ1

In which way does a balanced chemical equation demonstrate the conservation of matter?
O A. It shows the same number of atoms of each element on both sides of the equation.
B. It shows how the atoms of an element can appear on only one side of the equation.
C. It shows how matter can be both created and destroyed by a chemical reaction.
D. It shows the same number of molecules of each compound on both sides of the equation.
Answers
Answer:
A. It shows the same number of atoms of each element on both sides of the equation.
Explanation:
A balanced chemical equation demonstrates the conservation of matter by showing the number of atoms of each element on both sides of the equation or expression.
According to the law of conservation of matter, matter is neither created nor destroyed in the course of a chemical reaction but atoms are rearranged.
Based on this premise, the number of atoms or moles on both sides of the expression must be equal. If there are 3 atoms of carbon on one side, the product must also reflect 3 atoms of carbon.Which of the following are characteristics of ionic
compounds?
Answers
Answer:
Ionic compounds have high melting points.
Ionic compounds are hard and brittle.
Ionic compounds dissociate into ions when dissolved in water.
Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not.
Explanation:
What is the total charge of an iron nucleus
Answers
I have a balloon that has a volume of 0.5 L at a pressure of 0.5 atm. What is the new volume at a pressure of 1 atm?
I have a container at a volume of 2 L and at a temperature of 125 C. What is the new temperature of the container at a volume of 2 L?
A sample of helium gas in a balloon is compressed from 4.0 L to 2.5 L at a constant temperature. If the initial pressure was 3.0 atm at 4.0 L, what is the new pressure at 2.5 L?
A container has 50 mL of nitrogen at 25 C. What will be the volume if the new temperature if 60 C?
Answers
1)The new volume at a pressure of 1 atm is 0.25 L.
2)The new temperature of the container at a volume of 2 L is approximately 398°C.
3)The new pressure at 2.5 L is approximately 4.8 atm.
4)The new volume at a temperature of 60°C is approximately 55.8
1)To solve these gas law problems, we can use the ideal gas law equation, which states:
PV = nRT,
where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is temperature in Kelvin.
Balloon volume at a pressure of 0.5 atm:\(V_1\) = 0.5 L, \(P_1\)= 0.5 atm.
New volume at a pressure of 1 atm:\(P_2\) = 1 atm.
We can use the relationship\(P_1V_1 = P_2V_2\) to find the new volume (\(V_2\)).
(0.5 atm)(0.5 L) = (1 atm)(\(V_2\))
\(V_2\) = 0.25 L.
Therefore, the new volume at a pressure of 1 atm is 0.25 L.
2)Container volume: \(V_1\) = 2 L, \(T_1\)= 125°C.
New temperature at the same volume: \(V_2\) = 2 L.
We can use the relationship\(V_1\)/\(T_1\) = \(V_2\)/\(T_2\) to find the new temperature (\(T_2\)).
(2 L)/(125 + 273) K = (2 L)/(\(T_2\) + 273) K
Solving for\(T_2\), we get \(T_2\) ≈ 398°C.
Therefore, the new temperature of the container at a volume of 2 L is approximately 398°C.
3)Initial volume: \(V_1\)= 4.0 L, \(P_1\) = 3.0 atm.
Final volume: \(V_2\) = 2.5 L.
Since the temperature (T) is constant, we can use the relationship \(P_1\)\(V_1\) = \(P_2V_2\) to find the new pressure (\(P_2\)).
(3.0 atm)(4.0 L) = (\(P_2\))(2.5 L)
\(P_2\) ≈ 4.8 atm.
Therefore, the new pressure at 2.5 L is approximately 4.8 atm.
4)Initial volume: \(V_1\)= 50 mL, \(T_1\) = 25°C.
New temperature: \(T_2\) = 60°C.
We need to convert the temperatures to Kelvin.
\(T_1\)= 25 + 273 = 298 K, \(T_2\) = 60 + 273 = 333 K.
We can use the relationship \(V_1/T_1 = V_2/T_2\) to find the new volume (\(V_2\)).
(50 mL)/(298 K) = (\(V_2\))/(333 K)
\(V_2\) ≈ 55.8 mL.
Therefore, the new volume at a temperature of 60°C is approximately 55.8
Know more about volume here:
https://brainly.com/question/27710307
#SPJ8
ILL GIVE YOU A CROWN
Which farming method would mostly likely prevent soil erosion?
A.
contour plowing, to slow down water flow
B.
maximum tillage, to continually rotate topsoil
C.
planting one type of crop, to eliminate plant variety
D.
adding clay to the soil, to increase drainage

Answers
Answer:
A
Explanation:
Which of the following is the best explanation for melting? Circle or highlight the correct choice. A. Particles heat up, losing kinetic energy, and spread further apart so they are less attracted. B. Particles heat up, gaining kinetic energy, and spread further apart so they are less attracted. C. Particles cool down, losing kinetic energy, and get closer so they are more attracted. D. Particles cool down, gaining kinetic energy, and get closer so they are more attracted.
Answers
Write down the names of the elements

Answers
Answer:
Carbon and hydrogen
Explanation:
C = Carbon
H = Hydrogen
Draw the structures of methyl oleate and propylene glycol. Which one is more polar, and how can you tell
Answers
1234567891011121314151617181920