What is the molecular formula for a compound that is 40.0% C, 6.6% H, and 53.4% O and the molar mass is 120.0g/mol?

Answers
Answer:
molecular formula of the compound is C₄H₈O₄
Explanation:
First the empirical formula of the compound is determined:
Divide the percentage masses by the molar mass of each element to determine the mole ratio;
Carbon, C: 40/12 = 3.33
Hydrogen, H: 6.6/1 = 6.6
Oxygen, O: 53.4 / 16 = 3.3
dividing through with the smallest ratio to obtain the simplest whole number mole ratios
C = 3.3/3.3 = 1
H = 6.6/3.3 = 2
O = 3.3/3.3 = 1
Therefore, the empirical formula is CHO
molecular formula/mass = n (empirical formula/mass)
120 g/mol = n( 12 + 1 * 2 + 16)
30n = 120
n = 4 therefore, the molecular formula = 4(CH₂O)
molecular formula of the compound is C₄H₈O₄
Related Questions
An ionic compound contains only a metal, M, and bromine. If analysis indicates that a 3.44 g sample of the compound contains 2 g of M, what mass of Br in g is contained in 400 g of the mineral?
Enter your answer in decimal format with three decimal places and no units.
Answers
The mass of Br in 400 g of the mineral is 168.605 g.
To find the mass of Br in 400 g of the mineral, we first need to find the mass of M in 3.44 g of the compound.
We know that the sample contains 2 g of M, so the remaining mass (3.44 g - 2 g) must be Br.
This means that the compound is made up of 2 g M and 1.44 g Br.
To find the mass of Br in 400 g of the mineral, we can set up a proportion:
1.44 g Br is to 3.44 g compound as x g Br is to 400 g mineral.
Simplifying this proportion, we get:
1.44/3.44 = x/400
Solving for x, we get:
x = (1.44/3.44) * 400 = 168.605
Therefore, the mass of Br in 400 g of the mineral is 168.605 g, rounded to three decimal places.
For more questions on mass,
https://brainly.com/question/24191825
#SPJ11
A sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy of combustion of graphite. The temperature of the calorimeter increases from 26.74 °C to 27.93 °C. What is the heat capacity of the calorimeter and its contents?
Answers
Answer:
The correct answer is 15.54 kJ per degree C.
Explanation:
The enthalpy change for one mole of a substance, which combines or burns with the oxygen under the standard conditions, that is, at 25 degree C and 1 bar pressure is known as the standard molar enthalpy of combustion. The amount of heat transferred can be calculated by using the formula, q = mcΔT -------------(i)
Here q is the amount of heat transferred, c is the specific heat, ΔT is the change in temperature, and m is the mass of the substance. As in case of bomb calorimeter, mass if considered constant, thus, for calorimeter the equation mentioned will become, q = cΔT ---- (ii)
The standard molar enthalpy of combustion for carbon is -393.5 kJ/mol, that is, -393.5 kJ per mole of heat is generated by burning one mole of carbon. The molecular mass of carbon is 12 gram per mole.
Thus, the number of moles of carbon equivalent to 0.562 grams of carbon can be determined as,
Number of moles of carbon = mass / molecular mas
= 0.562 grams / 12 gram per mole
= 0.047 mol
The heat generated by burning 0.562 grams or 0.047 mole will be,
q = ΔH° × number of moles
= (-393.51 kJ/mol) × 0.047 mol
= -18.49 kJ, the negative sign shows that the heat is produced.
To find heat capacity of calorimeter, put the value of q as -18.49 kJ, for ΔT as (27.93 °C - 26.74 °C) in the equation (ii)
18.49 kJ = c × (27.93 - 26.74)
c = 18.49 kJ/1.19 °C
c = 15.54 kJ/°C
30 treadmills to 36 elliptical machines
Answers
Answer:
what? that's 66 total, 6 more elliptical machines, a 1 to 1.2 ratio
but I don't know what else you would mean
Answer:
5:6
Explanation:
Hope You Enjoy !
Sandy heated 20 grams of liquid hydrogen peroxide until it was completely broken down into liquid water and oxygen gas. Which of these best describes the total mass of water and oxygen that was produced?
A more than 20 grams since heat was added
B 20 grams since no matter was added or removed
C less than 20 grams since oxygen gas is very light
D more than 20 grams since there are two new substances
Answers
Hope it helps!
In the given scenario, 20 grams since no matter was added or removed. The correct option is B.
What is matter?Matter is a substance composed of various particle types that occupies physical space and has inertia.
According to modern physics principles, each type of particle has a distinct mass and size.
The electron, proton, and neutron are the most well-known examples of material particles.
Whether they are living or non-living. Matter is significant because it comprises everything around us.
Matter cannot be created or destroyed, instead, it can only be transformed into a different form.
On Earth, matter exists as a solid, liquid, or gas. Atoms and molecules are tiny particles that make up solids, liquids, and gases.
In the given scenario, 20 grams were added or removed because no matter was added or removed.
Thus, the correct option is B.
For more details regarding matter, visit:
https://brainly.com/question/3764450
#SPJ2
What is the frequency of an x-ray wave with an energy of 2×10 to the power of -17 jewels in hertz
Answers
The frequency of an x-ray wave with an energy of 2×10^(-17) J in hertz is 3.01 × 10^(16) hz.
We can find the value of frequency of an x ray wave by using the relation between energy and frequency.
As we know that,
Energy is directly proportional to the frequency of the wave.
Mathematically,
E = h. f
Where,
E is the energy in joule
f is the frequency of the wave in hertz
h is the planck's constant
Given,
E = 2 × 10^(-17)J
h = 6.63 × 10^(-34) kg.m2/s
By substituting all the values, we get
2 × 10^(-17)J = 6.63 × 10^(-34) kg.m2/s × f
f = 2 × 10^(-17)J / 6.63 × 10^(-34) kg.m2/s
f = 3.01 × 10^(16) hz.
Thus, we concluded that the frequency of an x-ray wave with an energy of 2×10^(-17) J in hertz is 3.01 × 10^(16) hz.
learn more about frequency:
https://brainly.com/question/14419982
#SPJ1
AgNO3 solutions are often used to plate silver onto other metals. What is the maximum amount of silver in grams that can be plated out of 4.8 L of an AgNO3 solution containing 3.4 Ag by mass? ( Assume that the density of the solution is 1.01 g/mL)
Answers
In this question, we have to find the maximum amount of grams that can be plated, and the informations we have are:
AgNO3
4.8 L of AgNO3 solution
3.4% of Ag by mass
density = 1.01g/mL
Using density and the volume of the solution, we can find the total mass of the solution, using the density formula:
d = m/v
d is going to be 1.01g/mL
m = ?
v = it will be 4800 mL, since we need mL and we have 4.8 L
Adding these values to the formula
1.01 = m/4800
m = 4848 grams
Now, since we have only 3.4% of Ag, we use the value in grams of the total solution to find the final mass of Ag
4848 g = 100%
x grams = 3.4%
x = 164.832 grams of Ag is the maximum amount.
the nurse is aware that fluid replacement is a hallmark treatment for shock. which of the following is the crystalloid fluid that helps treat acidosis?
Answers
One of the hallmark treatments for shock is fluid replacement, and the nurse is aware of this. In order to treat acidosis, the crystalloid fluid that is commonly used is called lactated Ringer's solution.
Fluid replacement is a crucial aspect of managing shock, as it helps restore blood volume and improve tissue perfusion. The nurse recognizes the significance of fluid therapy in treating this condition. Acidosis, characterized by an imbalance in the body's pH levels, can be a complication of shock.
To address acidosis and restore the body's acid-base balance, a crystalloid fluid known as lactated Ringer's solution is commonly employed. Lactated Ringer's solution contains sodium, potassium, calcium, and lactate, which helps in correcting acidosis by providing bicarbonate precursors.
This fluid not only replenishes the intravascular volume but also aids in the restoration of pH levels, making it an appropriate choice for treating acidosis associated with shock.
Learn more about Ringer's solution here:
https://brainly.com/question/26722450
#SPJ11
When an ionic solid is added into a solvent, you can see that the ionic solid dissociates into its respective cations and anions. The solvent molecules immediately cluster around the ions. This is known as solvation. When the solvent is water, the solvation process is called hydration. Dissolution and hydration occur simultaneously, and salt is said to be dissolved in water. You can observe that some of the ions dissolved in the solution recrystallize or deposit to form the solid salt. When the rate of dissolution equals the rate of recrystallization (deposition), dynamic equilibrium is reached.
Answers
The order of the steps, from first to last, is as follows based on the information in the question:
1. Salt is broken down into cations and anions.
2. Anion hydration.
3. Cation hydration.
4. Cations and anions that have been dissolved start to settle as a solid salt.
5. The rate of dissolution and recrystallization are equal.
Without hydration of the ions, the cation and anion cannot get separated. The three processes of ion dissociation, cation hydration, and anion hydration must all take place at once.
Why water is an effective solvent?Water is a great solvent that can dissolve a wide variety of compounds due to its polarity and capacity to create hydrogen bonds.
Learn more about solvation here:
https://brainly.com/question/530845
#SPJ4
i need help with this guys

Answers
Give an example of a change that the ecosystem was able to recover from and return to equilibrium.
Answers
Answer:
The Fox is at the top of the food chain; so, the other animals could increase in population and the fox could return
Moderate drought is an illustration of a shift that the prairie ecosystem was able to adapt to and return to balance. The amount of water that is accessible to both plants and animals reduces during a drought, which affects both plant development and the number of herbivores.
A forest ecosystem can recover from fire because diverse creatures play distinct functions in various ways and locations. Many species' dispersed habitats enable them to resprout or grow from seeds that are ignited by fire, protecting populations from being entirely wiped off.
Ecosystems are living things that are susceptible to a wide range of abiotic and biotic perturbations brought on by the forces of nature and/or human activities.
To know more about ecosystems, visit;
https://brainly.com/question/30761411
#SPJ3
Acetylene gas (C2H2) used in welding, pro-
duces an extremely hot flame when it burns
in pure oxygen according to the reaction
2 C₂H2(g) +5 O2(g) 4 CO2(g) + 2 H₂O(g).
How much CO2 is produced when 40000 g
of C₂H2 burns completely?
Answer in units of g.
Answers
Taking into account the reaction stoichiometry, 135.38 grams of CO₂ is produced when 40000 g of C₂H₂ burns completely.
Reaction stoichiometryIn first place, the balanced reaction is:
2 C₂H₂ + 5 O₂ → 4 CO₂ + 2 H₂O
By reaction stoichiometry, the following amounts of moles of each compound participate in the reaction:
C₂H₂: 2 molesO₂: 5 molesCO₂: 4 molesH₂O: 2 molesThe molar mass of the compounds is:
C₂H₂: 26 g/moleO₂: 32 g/moleCO₂: 44 g/moleH₂O: 18 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
C₂H₂: 2 moles ×26 g/mole= 52 gramsO₂: 5 moles ×32 g/mole= 160 gramsCO₂: 4 moles ×44 g/mole= 176 gramsH₂O: 2 moles ×18 g/mole= 36 gramsMass of CO₂ formedThe following rule of three can be applied: if by reaction stoichiometry 52 grams of C₂H₂ form 176 grams of CO₂, 40000 grams of C₂H₂ form how much mass of CO₂?
mass of CO₂= (40000 grams of C₂H₂ ×176 grams of CO₂)÷52 grams of C₂H₂
mass of CO₂= 135.38 grams
Finally, 135.38 grams of CO₂ is formed.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
When an acid dissolves in water, what solute is present in the solution? This isn't the only solute, but it is the solute that is common to acids. H'ions water molecules acid molecules H' ions
Answers
When an acid dissolves in water, the solute present in the solution is \(H^{+}\) ions as they are released by the acid molecules. This is a characteristic of all acidic solutions.
What is the reaction between an acid and water?When an acid dissolves in water, the acid molecules ionize to release \(H^{+}\) ions (also known as hydronium ions, \(H_{3}O^{+}\)). The H+ ions are the solute present in the solution of an acid dissolved in water. . Some examples of acidic solutions are hydrochloric acid (HCl), sulfuric acid (\(H_{2}SO_{4}\)), and nitric acid (\(HNO_{3}\)).
The concentration of \(H^{+}\) ions in an acidic solution determines the acidity of the solution. Acids have a pH less than 7. The more the concentration of \(H^{+}\) ions, the stronger the acid is, and the lower the pH of the solution. The concentration of \(H^{+}\) ions in a solution can be measured using a pH meter or pH paper.
To know more about acidic solutions:
https://brainly.com/question/29437746
#SPJ11
Which of the following acids has the strongest conjugate base?
Question options:
a. HClO
b.HClO_4
c.HClO_2
d.HClO_3
e.HCl
Answers
Conjugate bases are defined as the molecules or ions that remain after acids donate a proton. Conjugate base strength is determined by the strength of the parent acid. As a result, strong acids generate weak conjugate bases, while weak acids generate strong conjugate bases.
The correct option is b. HClO_4.HClO4 is the strongest acid since it has the highest Ka. Because the conjugate base of HClO4 is ClO4, which is quite stable and can handle negative charges better than the others, it is the strongest conjugate base. HClO4 is the strongest acid among the options provided because it has the highest Ka. The conjugate base of HClO4 is ClO4, which is a very stable base because it can manage the negative charge better than any other options provided.
Learn more about Conjugate bases here ;
https://brainly.com/question/30086613
#SPJ11
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
Count the number of atoms in each formula. Type your answer in the box to the right of the formula.
1) AgNO2 =
2) 2Fe2O3 =
3) (NH4)3PO4 =
4) 3CaCl2 =
Answers
Answer:
1) 4
2) 8
3) 20
4) 5
What volume of a 2.0M NaOH(aq) is needed to completely neutralize 24 milliliters of 0.5M HCl(aq)?
Show numerical setup and answer.
Answers
Answer:
\(V_{base}=6.0mL\)
Explanation:
Hello there!
In this case, by considering that the reaction between sodium hydroxide and hydrochloric acid is in a 1:1 mole ratio of these two reactants, we are able to use the following equation relating the concentration and volume of each one:
\(M_{acid}V_{acid}=M_{base}V_{base}\)
In such a way, by solving for the volume of the base, we will obtain:
\(V_{base}=\frac{M_{acid}V_{acid}}{M_{base}} \\\\V_{base}=\frac{0.5M*24mL}{2.0M}\\\\V_{base}=6.0mL\)
Regards!
If lamp 1 is unscrewed from its holder, what will happen to lamp 2 and why?

Answers
Hope it help
Mark me as brainliest
Which best describes nuclear fusion? O A nucleus spontaneously splits and absorbs energy O Two nuclei spontaneously combine and absorb energy. O A nucleus collides with a neutron and splits, releasing energy. O Nuclei combine to form a heavier nucleus, releasing energy.
Answers
Answer: Nuclei combine to form a heavier nucleus, releasing energy.
Explanation: e.g two deuterium nucleus (Hydrogen-2 isotopes) forms an He nucleus and energy is released.
Nuclear fusion is when nuclei combine to form heavier nuclei, releasing energy.
What is Nuclear Fusion?Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles (neutrons or protons). The difference in mass between the reactants and products is manifested as either the release or absorption of energy.
The release of energy with the fusion of light elements is due to the interplay of two opposing forces: the nuclear force, a manifestation of the strong interaction, which holds protons and neutrons tightly together in the atomic nucleus; and the Coulomb force, which causes positively charged protons in the nucleus to repel each other.
Nuclear fusion is also observed in stars.
To learn more about, fusion:
https://brainly.com/question/16556922
#SPJ5
How do you give the truth value of a proposition?
Answers
To give the truth value of a proposition, evaluate its accuracy based on evidence and logical reasoning.
To determine the truth value of a proposition, you evaluate whether the proposition is true or false based on the given information or conditions. A proposition is a declarative statement that can be either true or false, but not both. Here are the steps to assign a truth value to a proposition:
Understand the proposition: Read the statement carefully to ensure you grasp its meaning and intent.Analyze the context: Consider the context in which the proposition is being evaluated. Any relevant background information or conditions should be taken into account.Evaluate the proposition: Assess the truthfulness of the statement based on available evidence, logical reasoning, or empirical observations. Determine if the proposition aligns with reality and if it can be verified or disproven.Assign truth value: After careful consideration, assign the appropriate truth value to the proposition. If the statement is consistent with reality or verified, it is considered true; otherwise, it is false.Remember that assigning truth values to propositions requires critical thinking, logical analysis, and the consideration of relevant information. Additionally, in certain contexts, a proposition might be undecidable or contingent, meaning its truth value cannot be definitively determined.
Learn more about Truth
brainly.com/question/30671942
#SPJ11
Scientific predictions can best be described as: a Problems you expect to identify b Questions you expect to ask c Experiments you expect to run d Evidence you expect to find
Answers
Answer:
Explanation:a
Scientific predictions are best described as consistent data that are presented in the form of if and then. It is a solution to the problem that may be identified. Thus, option a is correct.
What is scientific prediction?Scientific prediction is a statement that describes the events of the future that are not always correct or incorrect. In scientific research, the experiment is conducted based on the hypothesis to draw conclusions and results that are expected.
The expected results are said to be scientific predictions when they are represented in the form of "if and then" before the analysis of the experimental method and its result are done. It is an account of the events that may possibly take place under specific conditions.
Therefore, option A. scientific prediction is an expected problem.
Learn more about scientific predictions, here:
https://brainly.com/question/20101086
#SPJ2
Please help I'm not sure what the answers are
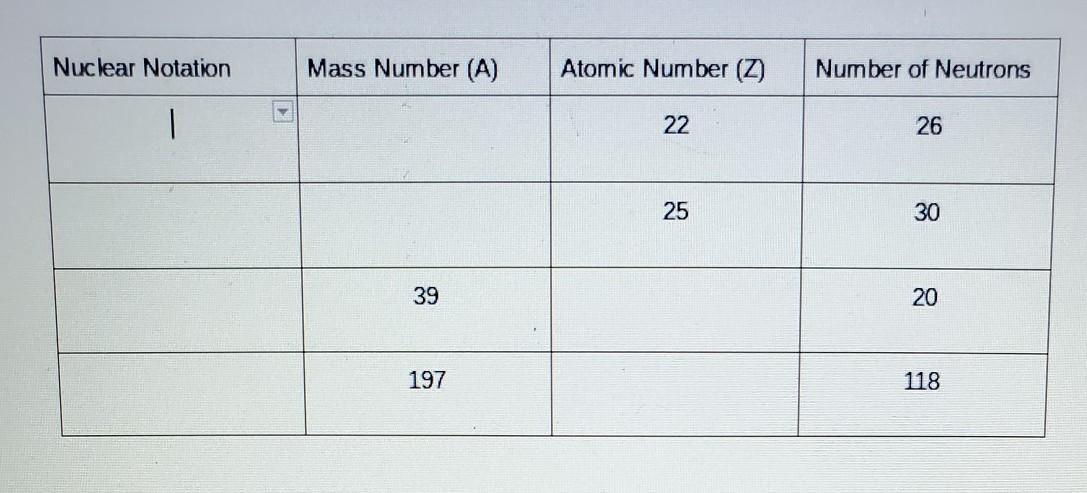
Answers
Answer:
An element's mass number (A) is the sum of the number of protons and the number of neutrons. The small contribution of mass from electrons is disregarded in calculating the mass number.
Question Which of the hypothesis tests listed below is a left-tailed test? Select all correct answers. Select all that apply OH 18, H₁ 19.3 H₂8. H:/8. OH-11.3 Hp
Answers
Among the given options, the tests that are left-tailed are H0:μ≥18, Ha:μ<18, H0:μ≥11.3, Ha:μ<11.3, and H0:μ≥3.7, Ha:μ<3.7.
In these tests, the null hypothesis (H0) states that the population mean (μ) is greater than or equal to a specific value, while the alternative hypothesis (Ha) suggests that the population mean is less than that value.
A left-tailed test is used when the alternative hypothesis suggests that the population parameter is less than a certain value.
This indicates a left-tailed test, where the critical region is in the left tail of the distribution. These tests focus on detecting a significant decrease or difference in the population mean.
To know more about the left-tailed test refer here :
https://brainly.com/question/31431592#
#SPJ11
Complete question :
Which of the hypothesis tests listed below is a left-tailed test? Select all correct answers.
Select all that apply: H0:μ≥18, Ha:μ<18 H0:μ≤19.3, Ha:μ>19.3 H0:μ=8, Ha:μ≠8 H0:μ≥11.3, Ha:μ<11.3 H0:μ≥3.7, Ha:μ<3.7
what is the slowest but most effective control for acid-base balance?
Answers
The slowest but most effective control for acid-base balance is the renal mechanism. This mechanism helps maintain pH levels in the body by removing excess acid or base from the bloodstream through urine production.
Acid-base balance-
Acid-base balance refers to the equilibrium between the acids and bases in the body. Maintaining an appropriate acid-base balance is crucial to optimal body function and good health. The pH scale is used to measure the acidity or alkalinity of a substance, with values ranging from 0 to 14. pH 7.4 is considered to be the normal pH level of blood.
The human body has three mechanisms for controlling acid-base balance, which include the buffer system, respiratory system, and renal system. The buffer system is the quickest and most efficient at restoring pH levels in the body but can only handle small changes. On the other hand, the respiratory system can compensate for larger changes in acid-base balance by regulating the amount of CO2 in the bloodstream.
Thus, the renal system is the slowest but most effective at controlling acid-base balance by eliminating excess acid or base from the bloodstream through urine production. The slowest but most effective control for acid-base balance is the renal mechanism. This mechanism helps maintain pH levels in the body by removing excess acid or base from the bloodstream through urine production.
Learn more about the renal mechanism from the given link-
https://brainly.com/question/32418480
#SPJ11
0/40
What is the volume of the two red spheres? (Hint: new
volume old volume)
6 ml
2 ml
4 ml
8 ml
Answers
Answer:
can you show me a picture of the two spheres thanks!!
What is the molarity of a sodium chloride solution that has 2.3 moles of NaCl in a volume of 350 mL?
Answers
Answer:
\(6.5714\text{M}\)
Explanation:
Number of moles of NaCl = 2.3 moles
Volume = 350 mL = \(350\times 10^{-3}\ \text{L}=0.35\ \text{L}\)
Molarity is given by
\(M=\dfrac{\text{Number of moles of NaCl}}{\text{Volume}}\)
\(\Rightarrow M=\dfrac{2.3}{0.35}\)
\(\Rightarrow M=6.5714\text{M}\)
Molarity of the solution is \(6.5714\text{M}\)
A liter of milk has a [H+] of about 2.51 × 10–7. (You may prefer to think of the hydronium ion concentration, [H3O+], as 2.51 × 10–7.) Use the formula for the calculation of pH provided and show each step as you calculate the pH of milk. In order to get full points you must show all the steps you take.
Answers
The pH of milk is approximately 6.6.
Steps
The formula for calculating pH is:
pH = -log[H+]
Given that [H+] of milk is about 2.51 × 10–7, we can substitute this value into the formula to calculate the pH:
pH = -log(2.51 × 10–7)
pH = -(-6.6)
pH = 6.6
Therefore, the pH of milk is approximately 6.6.
What is pH?A solution's acidity or basicity is determined by its pH. A pH of 7 indicates a neutral solution (equal concentration of H+ and OH- ions), a pH of less than 7 indicates an acidic solution (higher concentration of H+ ions), and a pH of greater than 7 indicates a basic or alkaline solution. It is defined as the negative logarithm of the hydrogen ion concentration [H+] in moles per liter (higher concentration of OH- ions).learn more about pH here
https://brainly.com/question/172153
#SPJ1
Where in nature would we find pure
sand that does not contain smaller or
larger particles?
Answers
Explanation:
Sand is a common material found on beaches, deserts, stream banks, and other landscapes worldwide. In the mind of most people, sand is a white or tan, fine-grained, granular material. However, sand is much more diverse - even beyond the pink sand beaches of Bermuda or the black sand beaches of Hawaii.
review: which dye have we used that forms hydrogen bonds with cellulose?
Answers
To review the characteristics of different dyes and their interactions with cellulose. One dye that is known to form hydrogen bonds with cellulose is Direct Orange 26.
It is a water-soluble dye that can bind to cellulose fibers through hydrogen bonding interactions. The hydroxyl (-OH) groups present in cellulose can form hydrogen bonds with the dye molecules, leading to strong and stable coloration. The formation of hydrogen bonds between Direct Orange 26 and cellulose allows for good dye fixation and wash fastness on cellulose-based materials, such as cotton.
This property makes it suitable for dyeing cellulose fibers in textile and dye industry applications. It's important to note that there may be other dyes that also form hydrogen bonds with cellulose, as hydrogen bonding is a common interaction between polar molecules. However, Direct Orange 26 is one example of a dye known for its hydrogen bonding capability with cellulose.
Learn more about hydrogen bonds here
https://brainly.com/question/31139478
#SPJ11
Can a pure element be a compound? Why or why not?
Answers
Yes, compounds are pure substances as they are made of molecules that are formed by the union of atoms of different elements through chemical bonding. Hence, molecules are the fundamental unit of compounds. Each compound is made of only one type of molecule.
The pH scale was designed to make it convenient to express hydrogen ion concentrations that are small in aqueous solutions. The definiton of pH is in terms of base 10 logarithms.

Answers
Answer:
a. pH = 2.22.
b. [H+] = 2.588 x 10⁻⁴ mol/L.
Explanation:
Acids and Bases => Calculating pH of Acids and Bases.
As we saw before, the formulas to find the pH based on the hydrogen ion concentration [H+], and to find the hydrogen ion concentration [H+] based on the pH are the following, respectively:
\(\begin{gathered} pH=-log\lbrack H^+], \\ \\ [H^+]=10^{-pH}. \end{gathered}\)So let's see each case:
a. To find the pH of an H+ concentration of 6.02 x 10⁻³ mol/L we use the pH formula:
\(\begin{gathered} pH=-log\lbrack6.02\cdot10^{-3}], \\ \\ pH=2.220\approx2.22. \end{gathered}\)The answer would be that the pH is 2.22.
b. To find the H+ concentration of a pH of 3.587, we use the [H+] formula:
\(\begin{gathered} \lbrack H{}^+]=10^{-3.587}, \\ \\ [H^+]=2.5882\cdot10^{-4}\text{ mol/L}\approx2.588\cdot10^{-4}\text{ mol/L.} \end{gathered}\)The answer would be that the hydrogen ion concentration [H+] = 2.588 x 10⁻⁴ mol/L.