What is the molar mass of nitrogen gas, N2(g)? How many moles of nitrogen
molecules are in 56 grams? Explain.
Answers
Answer:
28g/mol
2 moles of nitrogen are present
Explanation:
The molar mass is defined as the mass of a chemical when 1 mole of this substance is present and is used to convert mass of the substance to moles or vice versa. Is widely used in stoichiometry problems.
1 atom of nitrogen has a molar mass of 14g/mol. N₂(g) -The molecule that contains 2 atoms of nitrogen- has a molar mass of 28g. That is 28g/mol.
56g of N₂ are:
56g * (1mol / 28g) =
2 moles of nitrogen are presentRelated Questions
Assertion (A): Slaking of lime is an exothermic and combination reaction. Reason (R): Quick lime reacts with water to produce slaked lime.

Answers
Assertion (A) : Slaking of lime is an exothermic and combination reaction. Reason (R ) : Quick lime reacts with water to produce slaked lime. Thus the correct answer is option A Both (A) and (R) are true and (R) is correct explanation of the assertion.
Why is the Assertion correct?Slaking of lime, which is the process of adding water to quicklime to produce slaked lime, is an exothermic and combination reaction.
Quicklime, also known as calcium oxide, is a highly reactive substance that reacts with water to produce slaked lime, which is calcium hydroxide.
Therefore, The reaction is exothermic because it releases heat, and it is a combination reaction because it involves the combination of two substances to form a new compound. The reason given in (R) correctly explains the assertion in (A).
Learn more about Assertion from
https://brainly.com/question/26115325
#SPJ1
See full question below
Assertion (A) : Slaking of lime is an exothermic and combination reaction.
Reason (R ) : Quick lime reacts with water to produce slaked lime.
A
Both (A) and (R) are true and (R) is correct explanation of the assertion.
B
Both (A) and (R) are true but (R) is not the correct explanation of the assertion.
C
(A) is true but (R) is false.
D
(A) is false but (R) is true.
Fumarase catalyzes the conversion of fumarate to L-malate. It has a Km of 2.0 mM for fumarate, and a Vm of 2.6 mmol-min-1 per mg enzyme. What would be the rate of the reaction when the fumarate concentration is 1.2 mM and the amount of enzyme is 100 micrograms
Answers
The rate of the reaction when the fumarate concentration is 1.2 mM and the amount of enzyme is 100 micrograms is approximately 0.975 mmol-min-1 per mg enzyme.
The rate of the reaction can be calculated using the Michaelis-Menten equation:
\(V = (Vm * [S]) / (Km + [S])\)
Where:
V is the rate of the reaction
Vm is the maximum velocity (2.6 mmol-min-1 per mg enzyme)
[S] is the substrate concentration (1.2 mM)
Km is the Michaelis constant (2.0 mM)
By substituting the values into the equation, we can find the rate of the reaction:
V = (2.6 mmol-min-1 per mg enzyme * 1.2 mM) / (2.0 mM + 1.2 mM)
V = (3.12 mmol-min-1 per mg enzyme) / (3.2 mM)
V ≈ 0.975 mmol-min-1 per mg enzyme
Therefore, the rate of the reaction when the fumarate concentration is 1.2 mM and the amount of enzyme is 100 micrograms is approximately 0.975 mmol-min-1 per mg enzyme.
To know more about rate of the reaction, refer
https://brainly.com/question/24795637
#SPJ11
is carbon dioxide from a fire extinguisher a physical or chemical change?
Answers
Answer:
Fire is a chemical reaction between oxygen and the fuel. If you want to put out a fire, just get rid of one of those three things – fuel, oxygen or heat. ...It also prevents oxygen from reaching the fuel. Most fire extinguishers work by separating the fuel from the oxygen.
Explanation:
Answer:
It is a chemical change
Explanation:
ygyjhu went to the store and bumped into afghsiugshjkds
Answers
Answer:
well dont hurt yourself
Explanation:
its not very good to
2. What changes when an ion is made from an atom?
Answers
Answer:
When ions are made of a single atom, such as Li+1, they are called monatomic ions
Explanation:
Any atom or molecule with a net charge, either positive or negative, is known as an ion. An ion consisting of a single atom is a monoatomic ion; an ion consisting of two or more atoms is referred to as a polyatomic ion.
Which factors are needed for organisms to live earth
Answers
Answer: sunlight, water, air, habitat, and food.
Explanation: we are all living organisms and we all have our five basic necessities for survival; sunlight, water, air, habitat, and food.
how can you blance it and make it equal on both sides
2H2+o2=2H2o blance it
Answers
Answer:
it have been already balanced
2H2 + O2 = 2H2O.
Where do you think the energy we get in our lives come from?
Answers
As humans we get our energy from other sources such as plants or even other animals. This is by consuming nutrients such as protein, which is converted into energy.
hope this helps! :)
can you help me with this screenshot thank you and I will choose you for brainist if you are the first 3 that answer
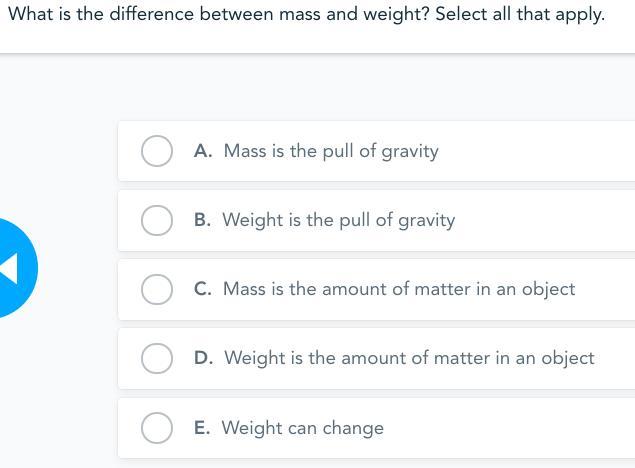
Answers
Your mass is the same no matter where you go in the universe your weight, on the other hand, changes from place to place. Mass is measured in kilograms even though we usually talk about weight in kilograms, strictly speaking it should be measured in newtons, the units of force.
Answer:
B. weight is the pull of gravity
C. mass is the amount of matter in an object
E. weight can change
Which layer is the oldest layer? How can you tell?
Answers
Answer:
The principle of superposition states that the oldest rock units are at rock bottom , and therefore the youngest are at the highest . Based on this, layer C is oldest, followed by B and A. So the full sequence of events is as follows: Layer C formed.
Explanation:
Answer:
the lowest one
the same way when you are stacking plates the oldest one is at the bottom and the most recently stacked one is at the top
Explanation:
Choose the reaction that illustrates AH°f for Mg(NO2)2.
O Mg(NO2)2(s) → Mg(s) + N2(g) + 402(g)
O Mg(s) + N2(g) + 2 O2(g) → Mg(NO2)2(s)
O Mg2+(aq) + 2 NO2-(aq) → Mg(NO2)2(aq)
O Mg(NO2)2(aq) → Mg2+(aq) + 2 NO2-(aq)
Answers
The correct reaction that illustrates AH°f for Mg(NO2)2 is:
Mg(s) + 2 NO2(g) + 1/2 O2(g) → Mg(NO2)2(s)
This reaction shows the formation of one mole of solid Mg(NO2)2 from its constituent elements in their standard states, with all reactants and products in their standard states at 25°C and 1 atm pressure. This is the definition of AH°f or the standard enthalpy of formation.
The other reactions listed involve either the decomposition of Mg(NO2)2 or the dissolution of its ions in water, which do not directly measure AH°f.
To Know more about measure visit;
https://brainly.com/question/3049557
#SPJ11
James Chadwick's experimental work in 1932
provided evidence for the existence of
within the nucleus. What one
word completes the sentence?
|
Answers
provided evidence for the existence of Neutrons
within the nucleus.
James Chadwick's experimental work in 1932
After conducting experiments for just two weeks, Chadwick produced a report in February 1932 titled "The Possible Existence of a Neutron" in which he made the argument that the neutron, not gamma ray photons, was the most likely explanation for the unexplained radiation.
James Chadwick, an English physicist, wasn't able to establish its reality until 1932. Instead of using gold foil, Chadwick modified Rutherford's experiment by using a sheet of beryllium and a paraffin block. He made the proton-sized neutral particle, today known as the neutron, in the process.
To learn more about : James Chadwick's
Ref: https://brainly.com/question/28207944
#spj2
what regulates the flow of chilled water through the cooling coil
Answers
The flow of chilled water through the cooling coil is regulated by a control valve.
In HVAC systems, the flow of chilled water through the cooling coil is regulated by a device called a control valve. The control valve is responsible for adjusting the flow rate of chilled water based on the cooling demand of the system. It ensures that the right amount of chilled water is supplied to the cooling coil to maintain the desired temperature in the conditioned space.
The control valve is typically controlled by a building automation system or a thermostat. These devices monitor the temperature in the conditioned space and send signals to the control valve to open or close. When the temperature rises above the set point, the control valve opens to allow more chilled water to flow through the cooling coil, cooling the air. Conversely, when the temperature falls below the set point, the control valve closes to reduce the flow of chilled water.
Learn more:About regulate here:
https://brainly.com/question/31313597
#SPJ11
The flow of chilled water through the cooling coil is regulated by a control valve. This valve adjusts the flow rate based on the cooling needs of the system.
A thermostat or temperature sensor provides signals to the control valve, which opens or closes accordingly.
When the temperature exceeds the desired setpoint, the control valve opens, allowing more chilled water to pass through the cooling coil.
This increases cooling capacity and lowers the air or space temperature.
Conversely, the control valve closes when the temperature reaches or falls below the set point, reducing chilled water flow.
The control valve ensures precise temperature control and efficient cooling operation in the system.
Read more about the Flow of chilled.
https://brainly.com/question/33293251
#SPJ11
what is the probability that an electron in the ground state of hydrogen will be found inside the nucleus griffiths
Answers
The probability that an electron in the ground state of hydrogen will be found inside the nucleus Griffiths are not higher than the outside nucleus.
Griffiths is the bohr's radius. According to bohr's, the probability that an electron in the ground state of hydrogen will be found inside the radius of nucleus. It is obtained by the expression,
P(r)= 1-e-2x (x+2x+2x2)
where x is equals to na .and n is the bohr's radius.
According to the Schrodinger's wave equation, the probability of an electron in the ground state of hydrogen will be found inside the nucleus Griffiths is not higher than the outside nucleus..as the volume of the nucleus is very small and they are similar to other volume that present outside the nucleus. It depends on the energy of the electron.
To learn more about Bohr's radius please visit:
https://brainly.com/question/14651996
#SPJ4
help this is for my cousin lol

Answers
Answer:
For camouflage, to blend in with their surroundings.
Explanation:
The solid, golden brown fur coat of the lion acts as camouflage and helps it hide to catch its prey, in low-lying grasses of the African savanna.
Iodine is prepared both in the laboratory and commercially by adding Cl2(g) to an aqueous solution containing sodium iodide. 2NaI(aq)+Cl2(g)⟶I2(s)+2NaCl(aq) How many grams of sodium iodide, NaI, must be used to produce 87.9 g of iodine, I2?
Answers
Answer:
\(m_{NaI}=104gNaI\)
Explanation:
Hello there!
In this case, given the balanced chemical reaction, it is possible to evidence that the mole ratio of sodium iodide to iodine is 2:1 and the molar masses are 149.89 and 253.81 g/mol respectively; in such a way, we write the following stoichiometric setup in order to obtain the required grams of sodium iodide:
\(m_{NaI}=87.9gI_2*\frac{1molI_2}{253.81gI_2}*\frac{2molNaI}{1molI_2}*\frac{149.89gNaI}{1molNaI} \\\\m_{NaI}=104gNaI\)
Regards!
17. the binding of the amino acid in aminoacyl-trna is a (n) a. amide c. hemiacetal b. ester d. ether
Answers
The binding of the amino acid in aminoacyl-tRNA involves the formation of an ester bond. Option b
Aminoacyl-tRNA is a complex molecule that plays a crucial role in protein synthesis. It consists of a tRNA molecule covalently linked to an amino acid. The amino acid is attached to the 3' end of the tRNA molecule through an ester bond.
An ester bond is formed between the carboxyl group (-COOH) of the amino acid and the hydroxyl group (-OH) of the ribose sugar at the 3' end of the tRNA molecule. This ester bond is also referred to as an ester linkage. The formation of the ester bond is catalyzed by the enzyme aminoacyl-tRNA synthetase.
The ester bond in aminoacyl-tRNA is essential for protein synthesis. During translation, the aminoacyl-tRNA molecule carries the specific amino acid to the ribosome, where it is incorporated into the growing polypeptide chain. The ester bond is later hydrolyzed, releasing the amino acid for further use in protein synthesis.
In summary, the binding of the amino acid in aminoacyl-tRNA involves the formation of an ester bond between the carboxyl group of the amino acid and the hydroxyl group of the ribose sugar in the tRNA molecule.
Option b
For more such question on amino acid visit:
https://brainly.com/question/30265108
#SPJ8
How many molecules are in 32 grams of NH3?
Answers
Answer:
1.87897962011728
Explanation:
9. Sequence the following electron configurations in order from least to greatest
atomic number: Hint: Exponents
1. 1s 2s22p%3s23p6452
II.1s22s22p63523p64523d104p3
III. 1s22s22p%3s23p64523d104p6
1.1s22s22p3s23p4523d4
ol|ol|ol|O
CLEAR ALL
Answers
The sequence : 1,4,2,3
Further explanationIn an atom there are levels of energy in the shell and subshell
This energy level is expressed in the form of electron configurations.
So the electron configuration shows the number of electrons it has
Atomic number = number of electrons in neutral atoms
Charging electrons in the sub shell uses the following sequence:
1s², 2s², 2p⁶, 3s², 3p⁶, 4s², 3d¹⁰, 4p⁶, 5s², 4d¹⁰, 5p⁶, 6s², etc.
1. 1s² 2s²2p⁶3s²3p⁶4s² : 2+2+6+2+6+2=20
2. 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p³ = 2+2+6+2++6+2+10+3=33
3. 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶ = 2+2+6+2++6+2+10+6=36
4. 1s²2s²2p⁶3s²3p⁶4s²3d⁴ = 2+2+6+2++6+2+4=24
How many grams of NaOH are there in 700.0 mL of a 0.18 M NaOH solution? *
a. 3.5 g
b. 2.19 x 10^-3 g
c. 149
d. 1149
e. 5.049
Answers
Answer:
E. 5.049
Explanation:
Multiply the concentration by volume (in liters) first to get moles of NaOH. Then multiply by the molar mass of NaOH to convert to grams.
0.18 M • 0.7000 L = 0.126 mol NaOH
0.126 mol • 39.997 g/mol = 5.040 g --> The closest answer seems to be e. 5.049 g
The walls of the alveoli are composed of two types of cells, type I and type II. The function of type II is to ________.
Answers
Type II alveolar cells are critical for maintaining the structure and function of the alveoli and for ensuring efficient gas exchange in the lungs.
The walls of the alveoli in the lungs are composed of two main types of cells, type I and type II alveolar cells. Type II alveolar cells, also known as septal cells or Type II pneumocytes, have several important functions in the lungs, including:
Production of surfactant: Type II alveolar cells secrete a substance called surfactant, which helps to reduce surface tension in the alveoli and prevent their collapse during exhalation. This is crucial for maintaining efficient gas exchange in the lungs.
Stem cell function: Type II alveolar cells are also thought to act as stem cells in the lungs, helping to regenerate damaged or injured lung tissue.
Immune function: Type II alveolar cells can also act as immune cells in the lungs, playing a role in the body's defense against pathogens and other foreign substances.
To know more about alveoli. here
https://brainly.com/question/11720309
#SPJ4
Juan Carlos placed 35 grams of ke into a dry, 200-gram container. The top of the container was attached tightly. When the ice was completely melted, the student
A.35
B.165
C.200
D.235
20 POINTS!!
Answers
Juan Carlos placed 35 grams of ke into a dry, 200-gram container. The top of the container was attached tightly. The mass of liquid water in the container is the same as ice. Therefore, the option A is correct.
What do you mean by mass ?The amount of matter in a particle or object is represented by mass (symbolized m), which is a dimensionless quantity. The kilogram is the International System (SIstandard )'s unit of mass (kg).
Mass is a quantitative measure of inertia and a fundamental property of matter. It is the resistance that a body of matter offers to change in speed or position when force is applied. The smaller the change produced by an applied force, the greater the mass of the body.
Because the number of particles does not change during a change of state, mass does not change. It makes no difference whether a substance freezes, boils, melts, or sublimates.
Thus, option A is correct.
To learn more about the mass, follow the link;
https://brainly.com/question/19694949
#SPJ6
In Niels Bohr’s model of the atom, how are electrons configured?
Answers
In Niels Bohr’s model of the atom, electrons are configured in a series of concentric shells around the nucleus. The shells are numbered, with the shell closest to the nucleus being numbered one, and each succeeding shell numbered two, three, and so on.
The electrons in the innermost shell have the lowest energy, while those in the outermost shell have the highest energy. Each shell can hold a certain number of electrons. The first shell can hold up to two electrons, the second shell up to eight electrons, and the third shell up to 18 electrons. Electrons fill the shells in a specific order, following the Aufbau principle. The principle states that electrons will occupy the lowest available energy level before filling higher levels. Electrons in the same shell have the same energy. Electrons in different shells have different amounts of energy, which corresponds to the distance of the shell from the nucleus. When an electron absorbs energy, it can move to a higher energy level. When an electron loses energy, it can move to a lower energy level. Electrons can also move between atoms, which is the basis of chemical reactions.For such more question on concentric shells
https://brainly.com/question/13569827
#SPJ8
imagine you are frosting a cake apply pascal's law to using the bag of frosting what would happen when you squeeze the bag
Answers
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
What is an ideal gas?
O A. A substance whose molecules do not take up space or interact
with one another
O B. A substance that can exist in either the gaseous state or the liquid
state
C. A substance that simultaneously has properties of a liquid, gas,
and solid
D. A substance that expands to fill a container, creating pressure and
heat
Answers
Answer:
A substance whose molecules do not take up space or interact with one another
A substance whose molecules do not take up space or interact with one another, hence option A is correct.
What are molecules?Molecules are defined as the lowest fundamental unit of a chemical molecule that can participate in a chemical reaction is a group of bound atoms. The smallest identifiable unit into which a pure substance may be divided while retaining its composition and chemical properties is a molecule, which is a collection of two or more atoms.
A theoretical gas known as an ideal gas is made up of several randomly moving point particles with no interparticle interactions. Because it abides by the ideal gas law, a condensed equation of state, and is amenable to statistical mechanics analysis, the ideal gas concept is helpful. The relationship between pressure and volume in an ideal gas is described by the Ideal Gas Law.
Thus, a substance whose molecules do not take up space or interact with one another, hence option A is correct.
To learn more about molecules, refer to the link below:
https://brainly.com/question/19922822
#SPJ2
A solid produced by a chemical reaction in solution that separates from the solution is called?
Answers
A solid produced by a chemical reaction in solution that separates from the solution is called Precipitate.
Precipitate: a solid that forms from a chemical reaction taking place in solution and separates from the solution.
It can also be formed by passing a gas into an aqueous solution of a substance (like passing carbon dioxide into lime water).a solid created when a solution undergoes a change, frequently as a result of a chemical reaction or temperature shift that makes a solid less soluble. A precipitate in meteorology is either liquid or solid water (rain, snow, etc.)
The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the 'Supernate' or 'Supernatant'.
Numerous instances of mineral production in nature can be attributed to precipitation reactions, such as metal sulphide creation at so-called "black smokers," submarine vents.Therefore, the chemical reaction in solution that separates from the solution is called precipitate.
For more such questions on Precipitate.
https://brainly.com/question/18109776
#SPJ4
Type the formula of the ionic compound:
lead (IV) hypochlorite
Answers
The hypothetical conjugate acid of the ortho-plumbate(IV) ion, PbO44, which is present in substances like calcium orthoplumbate, Ca2PbO4, is lead(IV) hydroxide, Pb(OH)4, commonly known as ortho-plumbic acid.
Lead IV oxide is a sort of chemical, right?The inorganic substance with the formula PbO2 is called lead(IV) oxide. It is an oxide in which the oxidation state of the lead is +4. It is a solid that is dark brown in color and insoluble in water. There are two crystalline forms of it.
Is a lead covalent or ionic?Lead is a post-transition metal that is comparatively unreactive. The amphoteric property of lead and lead oxides, which react with acids and bases and have a tendency to create covalent bonds, exemplifies the material's weak metallic character.
To know more about conjugate acid visit:-
brainly.com/question/30164261
#SPJ1
What information does the rate constant give from the rate law?
A. It tells how much the rate of the reaction is affected by volume.
B. It tells how much the rate of the reaction is affected by
temperature.
C. It tells how much the reaction rate is affected by concentrations.
D. It tells how much the reaction rate is affected by activation energy.
Answers
Answer:
C
Explanation:
Because how would you know what the reaction rate is and how it is affected by the concentrations
The information that the rate constant give from the rate law is it tells how much the reaction rate is affected by activation energy.
Hence, Option (D) is correct answer.
What is Rate Law ?The rate law is also known as rate equation which is defined as the law which is used to predict the relationship between the rate of the reaction and the concentration of the reactants participating in it.
It is expressed as
Rate = k [A]ˣ [B]ⁿ
where,
k is rate constant
A is concentration of species A
x is order of reaction w.r.t A
B is concentration of species B
n is order of reaction w.r.t B
Thus from the above conclusion we can say that The information that the rate constant give from the rate law is it tells how much the reaction rate is affected by activation energy.
Hence, Option (D) is correct answer.
Learn more about the Rate Law here: https://brainly.com/question/7694417
#SPJ2
What are diatomic molecules and are they compounds? PLEASE ANSWER ASAP!!
Answers
Answer:
Diatomic molecules consist of two atoms that are chemically bonded. The two atoms can be the same or different chemical elements. As for whether or not they are compounds, there is not technically an answer. This is because all compounds are molecules, but not all molecules are compounds. For example diatomic molecules that comprise the chemical compounds nitric acid, carbon monoxide, and hydrogen chloride are made up of two different elements. As you can see, most diatomic molecules are not made up of the same kind of elements and not every diatomic molecule comprises a chemical compound.
hope this helps :)
Explanation: