Answers
The molar mass of ZnI2 is approximately 319.18 grams per mole.
To determine the molar mass of ZnI2 (zinc iodide), we need to know the atomic masses of zinc (Zn) and iodine (I) and their respective subscripts in the chemical formula.
The atomic mass of zinc (Zn) is approximately 65.38 grams per mole (g/mol), as found on the periodic table. The atomic mass of iodine (I) is approximately 126.90 g/mol.
Since the chemical formula of zinc iodide is ZnI2, it means there are two iodine atoms for every one zinc atom. Therefore, we multiply the atomic mass of iodine by 2.
Molar mass of ZnI2 = (atomic mass of Zn) + 2 × (atomic mass of I)
= 65.38 g/mol + 2 × 126.90 g/mol
= 65.38 g/mol + 253.80 g/mol
= 319.18 g/mol
Hence, the molar mass of ZnI2 is approximately 319.18 grams per mole.
For more questions on molar mass, click on:
https://brainly.com/question/837939
#SPJ8
Related Questions
What is the chemical formula for sulfuric acid?
Answers
A student places one piece of chalk into a bowl with vinegar and another into a bowl with water. The next day she observes that the chalk in the water is mostly unchanged but the chalk in vinegar has pieces missing.
Which option describes a process in nature similar to the experiment? A.
heavy rainfall causing a landslide
B.
rocks at the bottom of a waterfall
C.
acid rain falling on a limestone sculpture
D.
salt water waves rolling over a rocky shoreline
E.
moving water of a river bumping rocks against each other
Answers
Heavy rainfall causes a landslide. Hence, option A is correct.
What is a landslide?A landslide is defined as the movement of a mass of rock, debris, or earth down a slope.
Water can trigger landslides and mudslides because it alters the pressure within the slope, which leads to slope instability.
Consequently, the heavy water-laden slope materials (soil, rock, etc.) will succumb to the forces of gravity.
Hence, option A is correct.
Learn more about the landslide here:
https://brainly.com/question/16151931
#SPJ1
PLEASE HELP! 27 PTS!
please submit illustration!!
Illustrate how the Sun affects Earth.
Materials:
-Posterboard
-Crayons, markers, or colored pencils
Instructions:
Review the following list of topics and think about the Sun's role in each:
Earth's energy budget
the water cycle
Earth's temperature
electromagnetic spectrum
photosynthesis
support of life
Choose one or two of the topics above, or think of your own topic that involves the Sun's relationship with Earth, and create an illustration of it.
Be sure that your illustration presents:
the Sun's involvement or role
any benefits or effects on Earth, life on Earth, and/or Earth's natural processes
Be creative!
Answers
Photosynthesis is the primary process for which the sun is responsible on earth.
What effects does the sun have on the planet Earth?Our world is significantly impacted by the sun, which also affects weather, ocean currents, seasons, and climate, as well as enabling photosynthesis, which is essential for plant life. Life would not exist on Earth without the sun's heat and light.Life on Earth continues as a result of this process because plants use sun energy to manufacture their food, which is then consumed by herbivorous animals and perpetuates the food chain. We may infer that the sun is crucial to the globe and its inhabitants because without it, no food would be created by creatures, and as a result, no life would exist on the planet.For more information on photosynthesis kindly visit to
https://brainly.com/question/29775046
#SPJ1
A rigid, 26-L steam cooker is arranged with a pressure relief valve set to release vapor and maintain the pressure once the pressure inside the cooker reaches 150 kPa. Initially, this cooker is filled with water at 175 kPa with a quality of 10 percent. Heat is now added until the quality inside the cooker is 40 percent. Determine the exergy.
Answers
The minimum entropy change of the heat-supplying source is -0.87 kJ/K.
Initial entropy of the systemIn this case, given the initial conditions, we first use the 10-% quality to compute the initial entropy.
at initial pressure of 175 kPaS₁ = 1.485 + (0.1)(5.6865) = 2.0537 kJ/kg K
Final entropyThe entropy at the final state given the new 40-% quality:
pressure inside the cooker = 150 kPaS₂ = 1.4337 + (0.4)(5.7894) = 3.7495 kJ/kg K
Mass of the steam at specific volumem₁ = 0.026/(0.001057 + 0.1 x 1.002643) = 0.257 kg
m₂ = 0.026/(0.001053 + 0.4 x 1.158347) = 0.056 kg
minimum entropy change of the heat-supplying sourceΔS + S₁ - S₂ + S₂m₂ - S₁m₁ - sfg(m₂ - m₁) > 0
ΔS + 2.0537 - 3.7495 + (3.7495 x 0.056) - (2.0537 x 0.257) - 5.6865( 0.056 - 0.257) > 0
ΔS > -0.87 kJ/K
Thus, the minimum entropy change of the heat-supplying source is -0.87 kJ/K.
Learn more about entropy here: brainly.com/question/6364271
#SPJ1
Which pictures shows the proper structure of the water molecule? Click the picture to see each choice.


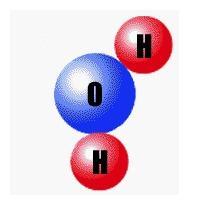

Answers
The third picture is the answer
Explanation:
there are 2 hydrogen atom 1 oxygen
The climate of an area can be different from its weather. Which of the following statements describes the climate of an area?
A. There should be heavy rains tomorrow morning.
B. The rains next week are expected to cause some flooding.
C. The average temperature from 1930–1996 was 23°C (74°F).
D. The high temperature on September 4, 2009, was 32°C (89°F)
Answers
Answer:
A
Explanation:
because on the first alphabet they've been specific of the weather partaking in the area
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
How many grams of NO will be produced, if 18.93g of H2O are also produced in the reaction below?

Answers
If 18.93g of H₂O are also produced in the reaction, then 21.04 grams of NO will be produced.
What is meant by reaction?Reaction is such a process that involves transformation of one or more substances into a new substances.
Assuming NH₃ is limiting reagent, let's calculate the amount of NH₃ from the given mass of H₂O produced:
Molar mass of H₂O is 18.015 g/mol;
So, 18.93 g H₂O ÷18.015 g/mol H2O= 1.0515 mol H₂O
As the mole ratio of NH₃ to H2O is 4:6, we can calculate the number of moles of NH₃ :
1.0515 mol H₂O × (4 mol NH₃ / 6 mol H₂O) = 0.701 mol NH₃
0.701 mol NH₃ × (4 mol NO / 4 mol NH₃ ) = 0.701 mol NO
0.701 mol NO × 30.01 g/mol NO = 21.04 g NO
Therefore, 21.04 grams of NO will be produced.
To know more about reaction, refer
https://brainly.com/question/25769000
#SPJ1
what are 2 ways that all hydrocarbons are alike?
Answers
Answer:
Composition: All hydrocarbons are made up of only two types of atoms: carbon and hydrogen. They are like building blocks that contain carbon and hydrogen stuck together.
Organic Nature: Hydrocarbons are special because they are part of a group of compounds that come from living things or things that were once alive. They have carbon and hydrogen in them, which is what makes them different from other types of compounds.
Explanation:
FeCl₃ has a van't Hoff factor of 3.400. What is the freezing point (in °C) of an aqueous solution made with 0.4100 m FeCl₃? (Kf for water is 1.860 °C/m)
Answers
FeCl₃ has a van't Hoff factor of 3.400. -3.858ºC is the freezing point (in °C) of an aqueous solution made with 0.4100 m FeCl₃.
What is freezing point?A liquid's freezing point is the temperature at which it turns into a solid. Similar to the melting point, the freezing point often rises with increasing pressure. In the case of combinations and for some organic substances, such as lipids, the freezing point usually below the melting point.
The first solid that forms when a combination freezes often differs in composition from the liquid, and the development of the solid alters the constitution of the remaining water, typically lowering the freezing point gradually.
∆T = i×m×Kf
i = van't Hoff factor = 3.400
m = molality = 0.4100 m
Kf = freezing constant = 1.860º/m
∆T = (3.400)(0.4100)(1.860)
∆T = 3.858º
Freezing point = 0º - 3.858º = -3.858ºC
Therefore, -3.858ºC is the freezing point.
To learn more about freezing point, here:
brainly.com/question/30168966
#SPJ1
What is the molar mass of iron (III) oxide?
Answers
Answer:
159.69 g/mol
Explanation:
Here's the answer hope it helps
A 75.0- mL
volume of 0.200 M
NH3
( Kb=1.8×10−5
) is titrated with 0.500 M
HNO3
. Calculate the pH
after the addition of 17.0 mL
of HNO3
.
Answers
Answer:
ok, here is your answer
Explanation:
i am going to solve this problem by using the ICE table method which is an easy method to determine the pH of a weak base with the given data of the problem.Given:Initial volume of NH3 solution (Vi) = 75.0 mLInitial concentration of NH3 solution (Ci) = 0.200 MInitial moles of NH3 solution (Ni) = Ci x Vi = 0.200 M x 75.0 mL = 0.0150 molesKb = 1.8 x 10^-5Moles of HNO3 added (n) = 0.500 M x 17.0 mL = 0.00850 molesVolume of NH3 solution after the addition of HNO3 (Vf) = 75.0 mL + 17.0 mL = 92.0 mLConcentration of NH3 solution after the addition of HNO3 (Cf) = Ni / Vf = 0.0150 moles / 92.0 mL = 0.163 MTo find the pH after the addition of 17.0 mL of HNO3, we need to use the ICE table method.ICE table method:Initial: NH3 + H2O ⇌ NH4+ + OH-Change: -x 0 +x +xEquilibrium: 0.0150 - x 0 x xKb = [NH4+][OH-] / [NH3]1.8 x 10^-5 = x^2 / 0.163Solving for x, x = 0.00171 M[OH-] = 0.00171 M[OH-] = Kw / [H3O+] = 1.0 x 10^-14 / [H3O+][H3O+] = 5.85 x 10^-12pH = -log[H3O+]pH = -log(5.85 x 10^-12)pH = 11.23Therefore, the pH after the addition of 17.0 mL of HNO3 is approximately 11.23.
mark me as brainliestHow many grams of the excess reactant remain after the limiting reactant is completely consumed? Express your answer in grams to three significant figures.
Answers
The question is incomplete, the complete question is;
One of the steps in the commercial process for converting ammonia to nitric acid is the conversion of NH3 to NO How many grams of NO and of H20 form? Enter your answers numerically separated by a comma. 4NH3(g) +502(g)------->4NO(g)+6H2O(g)
In a certain experiment, 1.10 g of NH3 reacts with 2.02 g of O2. How many grams of the excess reactant remain after the limiting reactant is completely consumed? Express your answer in grams to three significant figures.
Answer:
Mass of excess ammonia 0.034 g of ammonia
Mass of water formed= 1.37g
Mass of NO formed = 1.50g
Explanation:
The limiting reactant is the reactant that yields the least number of moles of product.
For NH3, molar mass of ammonia = 17g mol-1
Number of moles of ammonia reacted= 1.10g/17 gmol-1 = 0.065 moles of ammonia
According to the reaction equation;
4 moles of ammonia yields 4 moles of NO
Hence 0.065 moles of ammonia will yield 0.065 ×4/4 = 0.065 moles of NO
For oxygen, molar mass of oxygen gas = 32gmol-1
Number of moles of oxygen gas= 2.02g/32gmol-1 = 0.063 moles of oxygen
From the reaction equation;
5 moles of oxygen gas yields 4 moles of NO
0.063 moles of oxygen will yield 0.063 ×4 /5 = 0.050 moles of NO
Hence oxygen is the limiting reactant and ammonia is the excess reactant.
Amount of excess ammonia = Amount of ammonia - amount of oxygen
Amount of excess ammonia= 0.065-0.063= 2×10^-3 moles
Mass of excess ammonia = 2×10^-3 moles × 17 gmol-1 = 0.034 g of ammonia
Mass of NO formed is obtained from the limiting reactant. Since molar mass of is 30gmol-1. Then mass of NO formed = 0.050 moles of NO × 30gmol-1 = 1.50 g of NO
For water;
5 moles of oxygen yields 6 moles of water
Hence 0.063 moles of oxygen yields 0.063 × 6/5 = 0.076 moles of water
Molar mass of water = 18gmol-1
Hence mass of water = 0.076 moles × 18gmol-1 = 1.37g of water
A walnut stuck to a pin is burned beneath a can containing 100.0 grams of water at 21°C. After the walnut has completely burned, the
water's final temperature is a warmer 28°C. How much heat energy arose from the burning walnut?
2,000 joules
3,000 joules
5,000 joules
O 9,000 joules
Answers
Answer:5000 i think
Explanation:
How many milliliters of 12.0 M hydrochloric acid contain 3.646 g of HCl (36.46 g/mol)?
Group of answer choices
Answers
Answer:
8.3ml of 12M HCl contains 3.646 grams HCl
Explanation:
moles HCl = Molarity X Volume => Volume (Liters) = moles HCl/Molarity
Vol(L) = (3.646g/36.46g/mole)/12M = 0.0083Liter x 1000ml/L = 8.3ml
What happens to water when it is heated from 0 degrees celsius to 4 degrees celsius?
Answers
Explanation:
When water is heated from 0°C to 4°C, its volume decreases because its density increases and you can see this effect up to 4°C. Because the density of ice is maximum at 4 °C.
In other words, water heated from 0°C to 4°C, contracts continuously instead of expanding. It means its volume decreases up to 4°C.
Balance equation of gas burns carbon dioxide and water
Answers
gas = methane
burn with O₂ (oxygen)
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l)
Which of the following can be mixed in solution with NH3 to make a buffer?
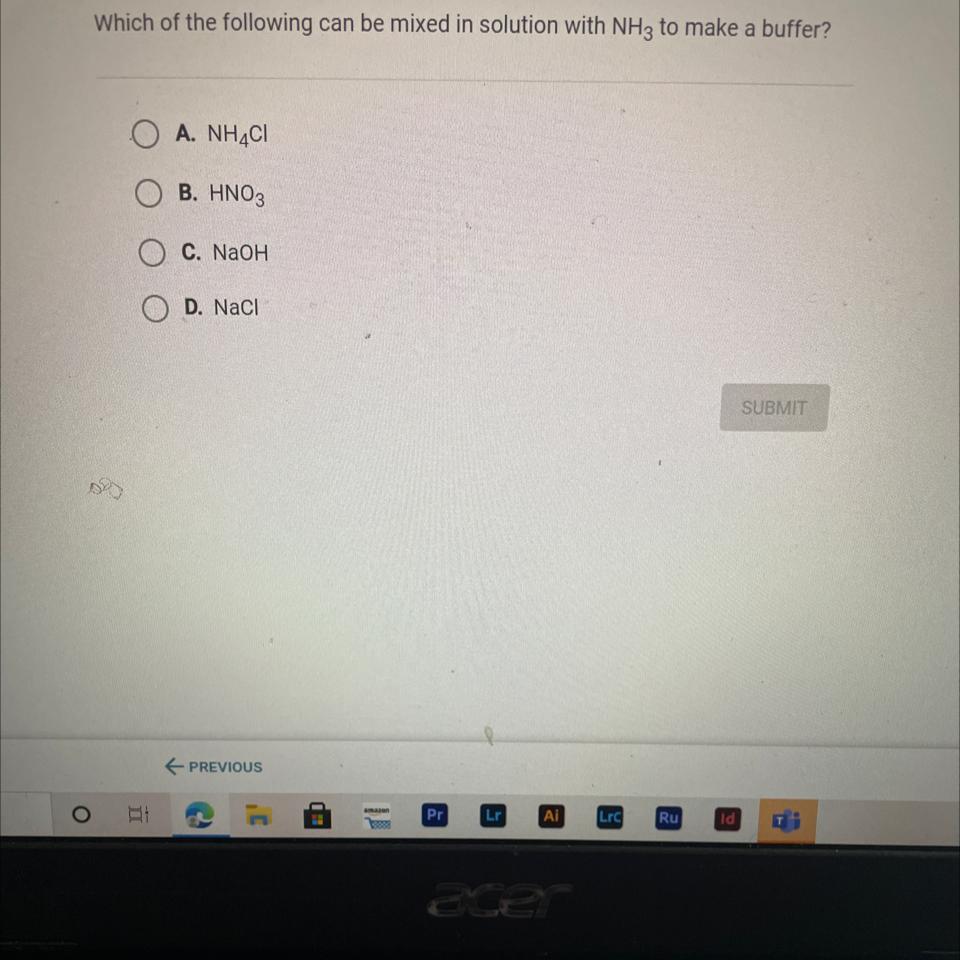
Answers
Answer:
c. NaOH
Explanation:
just took the quiz
What is the number of moles in 0.025g (NH4)2Cr2O7
Answers
Answer:
9.91 X 10^-5 or 0.000099 mol
Explanation:
What does it mean for an object to be dense?
Answers
Answer: Density is a measure
Explanation: The more squashed together an object’s particles are, the denser it is.
Which of the following is NOT a natural resource? Check all that apply.
1. Air
2. Water
3. Plastic
4. Sunlight
5. Cotton
6. Glass
7. Coal
8. Copper
Answers
While being made from natural resources, electricity does not constitute a natural resource because it goes through several procedures to produce it.
What are the seven categories of natural resources?Oil, coke, nat gas, metals, stone, or sand are examples of natural resources. Other natural resources include water, soil, sunlight, air, and so on. Natural resources have value because they enable life and provide for human needs.
A natural resource is land?Financially referred to it as land and raw materials, land resources (also known as natural resources) are found naturally within ecosystems that are mostly unaltered by civilization. A natural resource's biodiversity levels across different habitats are frequently used to describe it.
To know more about several visit:
https://brainly.com/question/13758372
#SPJ1
How do reactions involving gases affect the entropy of a system?
Answers
The wording of this question is a little weird, however, based on enthalpy and gases, converting from gases to liquids uses MORE energy than it uses to go from a gas to another gas. Depending on your state of matter, the amount of energy needed changes.
An excess of chromium metal is added to 500.0 mL of a 0.915 M AgNO3solution in a constant-pressure calorimeter. As a result of the reactionCr(s) + 2 AgNO3(aq)Cr(NO3)(aq) + 2 Ag(s)the temperature rises from 19.3 °C to 55.9 °C. Based on your previoustwo answers, calculate reaction (in J).Please help I don’t understand how i got it wrong :(

Answers
The enthalpy of the reaction is -164 kJ/mol.
What is the enthalpy of reaction?We know that the reaction that occurs between the chromium metal and the acid is an exothermic reaction thus there is an increase in the temperature of the system.
Number of moles of the silver nitrate solution is obtained from;
Volume * concentration
500/1000 L * 0.915 M = 0.46 moles
We can now assume that the density of the solution is 1 g/mL hence the mass of the solution is 500g. Let the specific heat capacity of the solution be 4.18 J/Kg/°C.
Then;
H = mcdT
H = Heat lost in the reaction
m = mass of the solution
c = specific heat capacity
dT = temperature change
H = 500 * 4.12 * ( 55.9 - 19.3)
= 75.4 kJ
The heat of reaction = 75.4 kJ/0.46 moles
= -164 kJ/mol
Let us recall that the negative simply means that heat was lost in the reaction.
Learn more about enthalpy:https://brainly.com/question/13996238
#SPJ1
Suppose you had four spoons the same size and shape made out of glass, plastic, steel, and wood. Which spoon handle would get hot the quickest when the spoons are placed in a pan of hot water?
Question 14 options:
A. steel spoon
B. plastic spoon
C. wood spoon
D. glass spoon
Answers
Answer:
A. steel spoon
Explanation:
A steel spoon will get hot the quickest from the different given spoon. This is because steel is predominantly made up of a metal which shows metallic properties such as heat conduction.
Metals have low specific heat capacity which is the amount of head needed to raise 1g of a substance by 1°C. By so doing, metals will conduct heat at a very fast rate. The delocalized electron around the central nucleus plays very important role.Other properties originating from metallic bonds are ductility, malleability, luster etc.
How many atoms are in 8g of Oxygen (O2)?
Answers
Answer:
There are 3.011 × 10^23 oxygen atoms
Describe another way you could collect or determine pressure data without using a gas pressure sensor, involving the same chemical reaction.
Answers
Another way you could collect or determine the pressure data without using a gas pressure sensor involving the same chemical reaction is using the Ideal Gas Equation.
A gas pressure sensor is a device used to monitor and collect data on the pressure changes and variations in a gas participating in a chemical reaction.
In a chemical reaction, the Ideal Gas Equation can be used to determine the pressure data without using a gas pressure sensor if the variables are known.
For example;
the numbers of moles(n) participating in the reaction, the volume(V), and;the temperature(T) at which the reaction is being carried outThe Ideal gas equation can be represented as:
PV = nRTwhere;
P = pressure data of the gasV = volume of the gasn = number of molesR = gas rate constantT = temperature of the gas.Therefore, we can conclude that the Ideal Gas Equation can be used to determine the pressure data without using a gas pressure sensor if the variables are known.
Learn more about the gas pressure sensor here:
https://brainly.com/question/18882857?referrer=searchResults
washing soda is a hydrate of sodium carbonate. a 2.714of washing soda is heated until a constant mass of 1.006g of NaNO3 is reached. what is the mass of water evaporated. what is the percent of water in the compound
Answers
37.06 percent is the percent of water in the compound
What is Washing Soda ?
Washing soda, also known as sodium carbonate, is a white crystalline powder that is commonly used as a cleaning and laundry aid. It is a strong alkaline substance and has a high pH, making it an effective cleaning agent for removing dirt, grease, and stains. Washing soda is often used as a household cleaner for tasks such as removing grease from ovens and pots and pans, cleaning tile and grout, and unclogging drains. In laundry, it can be used to remove stubborn stains, brighten clothes, and soften hard water. Washing soda can also be used in industrial applications, such as in the manufacture of glass, paper, and soap. It is a water-soluble compound and is generally considered safe for use, although it should be handled with care and not ingested, as it is a strong alkali and can be harmful if ingested in large quantities.
To learn more about Washing Soda; click the given link ;
https://brainly.com/question/10938067
#SPJ1
Calculator Reference
Fill in the blanks to balance the equation showing this reaction.
Type your answers in the boxes.
CO₂ + 4H₂S + O₂ → CH₂O +
IS +
H₂O
You may use the calculator.
Answers
Answer:
CO₂ + 4 H₂S + O₂ ---> CH₂O + 4 S + 3 H₂O
Explanation:
The unbalanced equation looks like this:
CO₂ + H₂S + O₂ ---> CH₂O + S + H₂O
Below is the amount of each element on each side of the reaction.
Reactants:
> 1 carbon, 2 hydrogens, 1 sulfur, 4 oxygens
Products:
> 1 carbon, 4 hydrogens, 1 sulfur, 2 oxygens
As you can see, there is a differing amount of hydrogens and oxygens on both sides. The balanced equation looks like this:
1 CO₂ + 4 H₂S + 1 O₂ ---> 1 CH₂O + 4 S + 3 H₂O
Below is the amount of each element on each side of the reaction.
Reactants:
> 1 carbon, 8 hydrogens, 4 sulfurs, 4 oxygens
Products:
> 1 carbon, 8 hydrogens, 4 sulfurs, 4 oxygens
Look at picture and answer will mark brainliest.
Answers
Answer:
A
Explanation:
Write a short essay about life in the Han Dynasty, comparing it to life today. Make sure to include key features:
-Family
-Government
-Social Structure
-Religion
-Trade
Answers
Answer:
Life in the Han Dynasty (206 BCE - 220 CE) differed significantly from today in family, government, social structure, religion, and trade. For example, the Han Dynasty emphasized a patriarchal family structure, where the eldest male held authority, and filial piety was highly valued. In contrast, contemporary societies embrace more egalitarian family dynamics with shared decision-making.
The government system of the Han Dynasty relied on a centralized bureaucracy and emphasized meritocracy, while modern societies often adopted democratic systems. Socially, the Han Dynasty followed a hierarchical model influenced by Confucian principles, whereas contemporary societies strive for greater equality and social mobility.
Religion in the Han Dynasty combined Confucianism, Taoism, and Buddhism, whereas modern societies exhibit diverse religious beliefs. Lastly, trade in the Han Dynasty thrived along the Silk Road, while modern trade was globally interconnected and facilitated by technological advancements. These differences highlight the evolution of society over time.
Explanation: