What is the maximum mass of ammonia that can be formed when 43.00 grams of nitrogen gas reacts with 10.62 grams of hydrogen gas according to the following equation? Please round your answer to the nearest 0.01 gram.
N2 + 3 H2 à 2 NH3
* thank you so much in advance!
Answers
54.88 grammes is the greatest mass of ammonia that may be produced. First, using the relative molar masses of the reactants, nitrogen gas (N2) and hydrogen gas (H2), the number of moles for each is calculated.
Next, it is found that nitrogen gas is the limiting reactant using the stoichiometry of the balanced equation. The stoichiometric ratio is used to convert the moles of nitrogen gas into moles of ammonia (NH3).
The final step in calculating the mass of ammonia is to multiply the moles of NH3 by their molar mass. When the maximal ammonia mass is rounded to the closest 0.01 gramme, 54.88 grammes are obtained.
Learn more about ammonia at :
https://brainly.com/question/29519032
#SPJ1
Related Questions
identify the most likely cause of earthquakes that occur in the area shown on the map

Answers
The most likely cause of earthquakes that occur in the area shown on the map is due to fault lines in the earth's crust.
What are earthquakes?Earthquakes are natural phenomena characterized by the shaking or trembling of the Earth's surface.
They occur due to the sudden release of energy in the Earth's crust along fault lines, which creates seismic waves that propagate through the Earth.
The Earth's crust is composed of several large tectonic plates that float on the semi-fluid layer of the Earth's mantle.
Learn more about earthquakes at: https://brainly.com/question/248561
#SPJ1
What is the area of the stratosphere with a high concentration of ozone?
A.) exosphere
B.) ionosphere
C.) O ozone layer
Answers
Answer:
C.) Ozone layer
Most ozone (about 90%) is found in the stratosphere, which begins about 10–16 kilometers (6–10 miles) above Earth's surface and extends up to about 50 kilo- meters (31 miles) altitude. The stratospheric region with the highest ozone concentration is commonly known as the “ozone layer” (see Figure Q1-2).
Which two particles are present in the nucleus of an atom?
Electrons and neutrons
Electrons and molecules
Protons and neutrons
Protons and electrons
Answers
Answer:
protons and neutrons are the answer
According to the graph,
what part(s) of the
reaction are present at
the beginning of the
reaction?
Concentration (M)
Reaction: 2A A₂
A. only the reactant, A
B. only the product, A:
C. Both the reactant (A) and product (A:)
D. You cannot determine from the graph.
Time (sec)
4

Answers
According to the graph, only the reactant A was present at the beginning of the reaction.
What does the graph show?The graph shows the concentration for the reactant A and the product that is A2. In this graph, the concentration is displayed on the vertical axis, while the horizontal axis shows the time.
In general terms, it can be observed that at the beginning only the reactant A is present, but as the reaction occurs the concentration of this reactant decreases, while the concentration of the product A2 increases.
Learn more about reactions in https://brainly.com/question/30464598
#SPJ1
34 points please help!!!!!!!!

Answers
Answer:
(i think) it is A difference in 1 unit is a 10X difference in concentration For example; a liquid with pH of 3 is 10X more acidic than a liquid with a pH of 4. Therefore, a liquid with a pH of 3 is stronger.
Explanation:
The redox potential indicator that detects the presence or absence of oxygen in the culture medium is called
Answers
The electrode can be used in conjunction with a dissolved oxygen meter to monitor oxygen levels in real-time and to control the oxygen supply to the culture medium to optimize growth conditions.
The redox potential indicator that detects the presence or absence of oxygen in the culture medium is called
"oxygen-sensitive electrode.
A redox potential indicator that detects the presence or absence of oxygen in the culture medium is known as an oxygen-sensitive electrode. It measures the partial pressure of oxygen (pO2) in a sample by transforming it into an electrical signal. It is made up of a platinum cathode and a silver anode, and it can be used in both aqueous and non-aqueous solutions.An oxygen-sensitive electrode is useful in a variety of applications, including fermentation processes and cell culture, where the presence or absence of oxygen affects the metabolic activity of the organisms in question. The electrode can be used in conjunction with a dissolved oxygen meter to monitor oxygen levels in real-time and to control the oxygen supply to the culture medium to optimize growth conditions.
To know more about electrode visit:
https://brainly.com/question/33425596
#SPJ11
pedro collected temperature data from the

Answers
The statement which have correct evidence supporting above is that electrical energy can be changed or converted into other form of energy.
Therefore, Option A is the correct option.
What is electrical energy?Electrical energy is defined as the energy which is derived from electric kinetic energy or potential energy of the charged particles.
In general, it is referred as the amount of energy which has been changed from electric potential energy. We can also define electrical energy as the energy which is generated by the movement of electrons from one position to another position. The movement of charged particles or electron through/along a medium which constitute current or electricity.
Unit of Electrical EnergyThe SI unit of Electrical Energy is watt-second.
The commercial unit of Electrical energy is kilowatt-hour.
1Kwh = 3.6 × 10^6 J.
Some example of Conversion of Electrical energy are as follow:
Fan - The electrical energy is converted into mechanical energy and heat energy.
Therefore, the electrical energy in light bulb is converted into light energy and heat energy.
Thus, we concluded that the electrical energy can be changed or converted into other form of energy.
learn more about Electrical energy:
https://brainly.com/question/12300421
#SPJ9
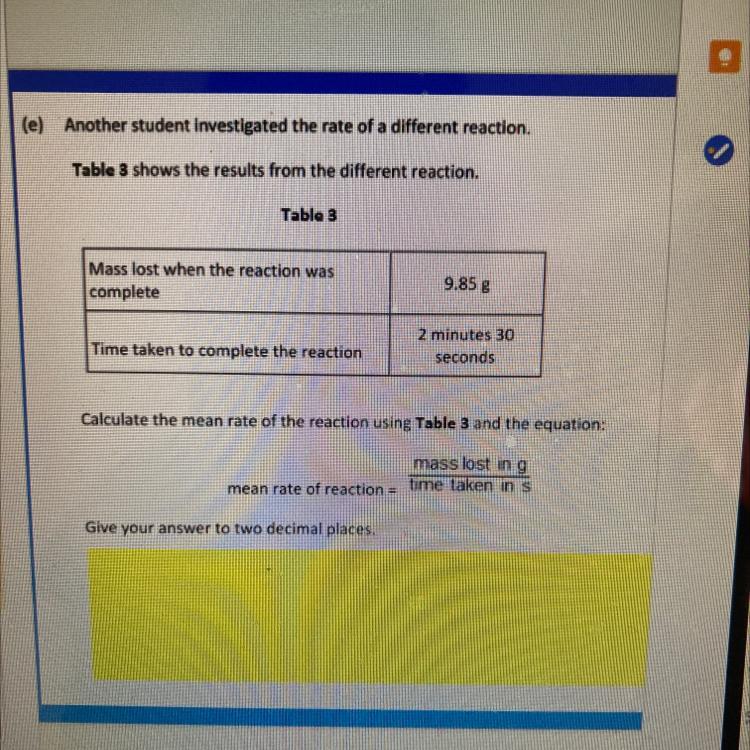
(e) Another student investigated the rate of a different reaction
Table 3 shows the results from the different reaction
Headings that you a
will appear here.
Table 3
Mass lost when the reaction was
complete
9.85 g
Time taken to complete the reaction
2 minutes 30
seconds
Calculate the mean rate of the reaction using Table 3 and the equation:
mass lost in g
mean rate of reaction time taken in s
Give your answer to two decimal places.
Answers
Answer:
0.07 g/s.
Explanation:
From the question given above, the following data were obtained:
Mass lost = 9.85 g
Time taken = 2 min 30 s
Mean rate =?
Next, we shall convert 2 min 30 s to seconds (s). This can be obtained as follow:
1 min = 60 s
Thus,
2 min = 2 × 60 = 120 s
Therefore,
2 min 30 s = 120 s + 30 s = 150 s
Finally, we shall determine the mean rate of the reaction. This can be obtained as illustrated below:
Mass lost = 9.85 g
Time taken = 150 s
Mean rate =?
Mean rate = mass lost / time taken
Mean rate = 9.85 / 150
Mean rate = 0.07 g/s
Therefore, the mean rate of the reaction is 0.07 g/s
Persamaan berikut menunjukkan tindak balas antara asid sulfurik dan kalium hidroksida.Berapakah isi padu larutan kalium hidroksida 0.5 mol dm-3 yang boleh meneutralkan 50.0 cm3 asid
sulfurik 0.5 mol dm-3?
H2SO4 + 2KOH -> K2SO4 + 2H2O
A 25.0 cm3
B 50.0 cm3
C 100.0 cm3
D 400.0 cm3
Answers
Explanation:
2 KOH (aq) + H2SO4 (aq) →K2SO4 (aq) + 2 H2O (l)
supposed chemists attempt to produce an element with atomic number 119 based on it’s likely position on the periodic table what would you expect it’s electronegativity to be? explain how you can make this prediction
Answers
An element with atomic number 119 will be an alkali metal with a +1 oxidation state which makes it highly electronegative.
What is Electronegativity?This is described as the tendency of the atom of an element to attract electrons so as to form a bond. This is done so that the elements can achieve a stable octet configuration.
On the other hand, if an element with atomic number 119 was present based on it’s likely position on the periodic table then it will most likely be an alkali metal with a +1 oxidation state and will be highly electronegative as it requires the loss of only one electron in other to achieve a stable configuration thereby making it highly reactive.
Read more about Electronegativity here https://brainly.com/question/18258838
#SPJ1
Farmer Brown is planting crops in his fields. He wants to prevent the topsoil from being blown away by the wind or washed away by water. Which sustainable farming practice should he use?
A) inter cropping
B) cover crops
C) crop rotation
D) tillage
Answers
What is the mole fraction of KCI in an
aqueous solution that contains
26.3% KCI (weight/weight %)?
Answers
Answer:
The mole fraction is the number of moles of something divided by the number of total moles present. First, let us work out the number of moles of KCl present. A 26.3% w/w solution would contain 26.3 g of KCl per 100 g of solution. Hence, 26.3 g of KCl is 26.374.5513 = 0.353 moles
Explanation:
please mark me as brainliest
find the ph of a mixture that is 0.020 m in hbr and 0.015 m in hclo4.
Answers
The pH of the mixture can be calculated using the equation: pH = -log[H+], where [H+] is the concentration of hydrogen ions in the solution.
To find the [H+] in the mixture, we need to first calculate the individual [H+] values for each acid, using the equation for the dissociation of acids in water:
HBr → H+ + Br-
HClO4 → H+ + ClO4-
For HBr, the [H+] is equal to the concentration of HBr, since it dissociates completely. So [H+] for HBr is 0.020 M.
For HClO4, we need to use the acid dissociation constant (Ka) to calculate the [H+]. Ka for HClO4 is 7.5 x 10^-1, so:
Ka = [H+][ClO4-] / [HClO4]
[H+] = sqrt(Ka[HClO4]) = sqrt(7.5 x 10^-1 x 0.015) = 0.049 M
To find the total [H+] in the mixture, we add the [H+] values for HBr and HClO4:
[H+] total = [H+] HBr + [H+] HClO4 = 0.020 + 0.049 = 0.069 M
Finally, we can use the equation pH = -log[H+] to find the pH:
pH = -log(0.069) = 1.16
Therefore, the pH of the mixture is approximately 1.16.
Visit here to learn more about pH:
brainly.com/question/12609985
#SPJ11
what volume (in ml) of 0.3850 m hcl is required to neutralize 60.00 ml of 0.7000 m koh
Answers
109.1 mL of 0.3850 M HCl is required to neutralize 60.00 mL of 0.7000 M KOH.
To calculate the volume of 0.3850 M HCl required to neutralize 60.00 mL of 0.7000 M KOH, the following is the solution:
First, calculate the amount of KOH that reacted with HCl using the following formula: Moles of KOH = Molarity x Volume = 0.7000 M x 60.00 mL = 0.04200 mol.
Now that the amount of KOH has been determined, it is time to determine how much HCl is needed. Since KOH and HCl react in a 1:1 ratio, the number of moles of HCl required is also 0.04200 mol.
Volume of HCl = Moles of HCl / Molarity. Hence, the volume of 0.3850 M HCl required to neutralize 60.00 mL of 0.7000 M KOH = 0.04200 mol / 0.3850 M = 109.1 mL.
Therefore, 109.1 mL of 0.3850 M HCl is required to neutralize 60.00 mL of 0.7000 M KOH.
To learn more about molarity, refer below:
https://brainly.com/question/8732513
#SPJ11
What is the surface tension and why is it important
Answers
Answer:
Surface tension is an important factor in the phenomenon of capillarity . Surface tension has the dimension of force per unit length, or of energy per unit area.
Explanation:
Ethylene, CH4, burns in oxygen to give carbon dioxide, CO2, and water. Write the equation for the reaction, giving molecular, molar, and mass interpretations below the equation.
Answers
The equation for the combustion reaction of ethylene (C2H4) with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O) is:
C2H4 + 3O2 -> 2CO2 + 2H2O
The balanced equation represents the stoichiometry of the combustion reaction. Here is the interpretation of the equation:
Molecular Interpretation:
1 molecule of ethylene reacts with 3 molecules of oxygen to produce 2 molecules of carbon dioxide and 2 molecules of water.
Molar Interpretation:
1 mole of ethylene reacts with 3 moles of oxygen to produce 2 moles of carbon dioxide and 2 moles of water.
Mass Interpretation:
The molar masses of the compounds involved are:
C2H4: 2(12.01 g/mol) + 4(1.01 g/mol) = 28.05 g/mol
O2: 2(16.00 g/mol) = 32.00 g/mol
CO2: 1(12.01 g/mol) + 2(16.00 g/mol) = 44.01 g/mol
H2O: 2(1.01 g/mol) + 16.00 g/mol = 18.02 g/mol
Therefore, when 28.05 grams of ethylene react with 96.00 grams of oxygen, they produce 88.02 grams of carbon dioxide and 36.04 grams of water.
The balanced equation C2H4 + 3O2 -> 2CO2 + 2H2O represents the combustion of ethylene with oxygen, resulting in the formation of carbon dioxide and water. The equation can be interpreted on a molecular, molar, and mass basis to understand the stoichiometry and quantities involved in the reaction. This information is useful for calculating reactant and product amounts, as well as for understanding the composition and yields of the combustion products.
To know more about ethylene visit:
https://brainly.com/question/14797464
#SPJ11
What process transfers water from the atmosphere to the hydrosphere
A evaporation
B runoff
C precipitation
D currents
Answers
Most of the pollution in the ocean comes from:
automobiles
industrial waste
carbon dioxide
acid rain
PLEASE HELP ASAP!!!
Answers
a certain radioactive element looses 20% of its mass per year, what is the half life of this substance
Answers
The half-life of the radioactive element is approximately 3.32 years.
The concept of half-life refers to the time it takes for half of the radioactive substance to decay. In this case, since the element loses 20% of its mass per year, we can calculate the half-life using the exponential decay formula.
If the substance loses 20% of its mass per year, it means that the remaining mass after each year is 80% (100% - 20%) of the previous year's mass. This can be expressed as a fraction: 0.8.
To find the number of years it takes for the mass to reduce to half, we can set up the following equation:
(0.8)^(number of years) = 0.5
Taking the logarithm of both sides, we have:
log(0.8) * (number of years) = log(0.5)
Solving for the number of years:
(number of years) = log(0.5) / log(0.8) ≈ 3.32 years
Therefore, the half-life of this radioactive element is approximately 3.32 years.
You can learn more about radioactive element at
https://brainly.com/question/23759636
#SPJ11
What is the density of a plastic ball that has a volume of 6cm3 and a mass of 12g?
Answers
Answer:
The answer is 2 g/cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\ \)
From the question we have
\(density = \frac{12}{6} \\ \)
We have the final answer as
2 g/cm³Hope this helps you
give the neutral formula unit for the combination of the following: calcium and no3–.
Answers
The neutral formula unit for the combination of calcium and NO₃⁻ is Ca(NO₃)₂. Calcium is a metal that belongs to group 2 of the periodic table, and it has a +2 charge. NO₃⁻ is a polyatomic ion that has a -1 charge.
When these two elements combine, they form an ionic compound through electrostatic attraction. The calcium cation and the nitrate anions combine in a 1:2 ratio to form the neutral compound Ca(NO₃)₂. This formula unit represents the simplest ratio of atoms in the compound and indicates that one calcium ion is combined with two nitrate ions.
So, the neutral formula unit for the combination of calcium (Ca) and nitrate (NO₃⁻) is Ca(NO₃)₂. In this compound, calcium has a charge of +2, while each nitrate ion has a charge of -1. To create a neutral formula unit, we need two nitrate ions for each calcium ion to balance the charges.
To know more about formula unit, refer
https://brainly.com/question/24529075
#SPJ11
Cat or Dog?
Zebra or Tiger?
China or NYC?
Sing or Dance?
Exercise or Yoga?
Gaming or Study?
Cooking or Reading?
:3 bye hope you have a lovely day!
Answers
Answer:
Dog
Zebra
Sing
Yoga
Gaming
Cooking
Can someone please solve this for me and explain it

Answers
83.3% yield
Explanation:
First, we need to convert 240 g of \(Fe_{2}O_{3}\) into moles:
\(240 \:g \:Fe_{2}O_{3} \:\times(\frac{1\:\text{mol}\:Fe_{2}O_{3}}{159.69\: \text {g}\:Fe_{2}O_{3}})\)
\(=1.50 \:\text{mol}\:Fe_{2}O_{3}\)
Next, find the theoretical Fe yield using molar ratios.
\(1.50 \: \text {mol} \: Fe_{2}O_{3}\: \times (\frac{2\: \text{mol} \: Fe}{1 \:\text{mol} \: Fe_{2}O_{3}})\)
\( = 3.00 \: \text{mol} \: Fe\)
Then convert this back into grams:
\(3.00 \: \text{mol} \:Fe \times (\frac{55.845 \: \text{g} \: Fe}{1 \: \text{mol} \: Fe}) = 168 \: \text{g} \: Fe\)
Note that actual yield is only 140 g Fe so percentage yield is
\(\dfrac{140\:\text{g}\:Fe}{168\:\text{g}\:Fe} \times 100\)%= 83.3%
83.3%
The answer is 83.3%
BRAINILIEST PLEASEThe Chemical Formula For Lead(II) Nitrite Is: Pb(NO2) 2 How Many Oxygen Atoms Are In Each Formula Unit Of Lead (II) Nitrite?
Answers
The number of oxygen atoms in each formula unit of lead nitrite is equal to four.
What is the formula unit?A formula unit can be used to represent the lowest whole number ratio of ions in an ionic compound. The formula mass of an ionic compound is equal to the sum of the atomic masses of the ions in the formula unit.
A formula unit can be described as an empirical formula of any covalent or ionic compound that can be used as an independent entity for stoichiometric calculations.
Given the chemical formula of the Lead(II) nitrite is Pb(NO₂)₂. The number of oxygen atoms in each formula unit is four.
Learn more about formula unit, here:
brainly.com/question/15971114
#SPJ1
Give one example of each of the following, that happens to us in our everyday life: Explain a bit about the science behind it, so for example, for melting you can say ice cream melting in your hand, which turns from a solid to a liquid, which is melting. If you are unsure please do not answer, though if you are confident please be free to do so! Have a wonderful day or night!
a) Melting:
b) Freezing:
c) Condensation:
d) Evaporation:
e) Sublimation.
Answers
a) Melting: An example of melting that occurs in our everyday life is when we heat butter on a stovetop.
b) Freezing: Freezing is the process in which a liquid transforms into a solid upon cooling.
c) Condensation: One example of condensation that we encounter regularly is when water droplets form on the surface of a cold drink on a hot day.
d) Evaporation: Evaporation is the process by which a liquid transforms into a gas or vapor.
e) Sublimation: Sublimation refers to the transformation of a substance directly from a solid to a gas without passing through the liquid state.
a) Melting: Butter is a solid at room temperature, but when heat is applied, it melts into a liquid. This change is a result of the increase in temperature, which provides enough energy to overcome the intermolecular forces holding the butter molecules together.
b) Freezing:Eventually, the temperature reaches the freezing point of water (0°C or 32°F), at which the water molecules slow down and arrange themselves into a regular, crystalline structure. This transformation from a liquid to a solid state is accompanied by the release of heat energy.
c) Condensation: As the temperature decreases, the air's capacity to hold moisture decreases, causing the water vapor in the air to condense into liquid water droplets. This process occurs due to the transfer of heat energy from the warm air to the cold surface, leading to the saturation of the air and the conversion of water vapor into liquid form.
d) Evaporation: As the sun's heat energy is absorbed by the water molecules on the clothes' surface, their kinetic energy increases, causing them to break free from the liquid phase and escape into the surrounding air as water vapor. This process occurs because the molecules at the liquid surface with sufficient energy can overcome the attractive forces within the liquid and enter the gas phase.
e) Sublimation: Sublimation refers to the transformation of a substance directly from a solid to a gas without passing through the liquid state. An example of sublimation is the process of dry ice (solid carbon dioxide) converting into carbon dioxide gas.
For more such questions on Freezing visit:
https://brainly.com/question/40140
#SPJ8
um.. i dont need help lol
Answers
Answer:
thats cool mate
Explanation:
hope ya have a good day, im answering just for the points tbh
Answer:
ello thats great free points then >:3
-XxanimexX
Which chemical leads to a pleasurable sensation when released in the brain, such as in response to nicotine?.
Answers
One of these neurotransmitters, dopamine, is released in the reward area of the brain and results in pleasurable feelings and an uplifted mood. More nicotine is required to feel good the more you smoke.
What is Nicotine ?The nightshade plant family naturally produces nicotine, an alkaloid that is widely used recreationally as a stimulant and anxiolytic. It is a medication meant to decrease withdrawal symptoms in smokers who desire to quit.
Nicotine is a highly addictive and dangerous drug. It might cause a rise in blood pressure, an increase in heart rate, and arterial constriction (vessels that carry blood). The hardening of artery walls, which may lead to a heart attack, is another potential effect of nicotine.Learn more about Nicotine here:
https://brainly.com/question/23382655
#SPJ4
Silver bromide is the photosensitive material in 35 mm photographic film. When monochromatic light falls on film, the photons are recorded if
they contain sufficient energy to react with silver bromide in the film. The minimum energy needed to do this is approximately 57.9 kJ/mol. What
is the wavelength of this energy in nm?
Answers
The minimum energy needed by the photons to react with silver bromide in the film is approximately 57.9 kJ/mol. Then, the wavelength is 206 nm.
What is wavelength?Wavelength of a wave is the distance between two consecutive crests or troughs of the wave.
To find the wavelength of the energy in nm, we can use the equation:
E = hc/λ
where E is the energy in joules, h is Planck's constant (6.626 x 10⁻³⁴ J s), c is the speed of light (2.998 x 10⁸ m/s), and λ is the wavelength in meters.
First, we need to convert the energy from kJ/mol to J/photon:
57.9 kJ/mol = 57.9 x 1000 J/mol / 6.02 x 10²³ mol^-1
= 9.626 x 10²⁰ J/photon
Now we can use the equation above to find the wavelength:
9.626 x 10²⁰ J/photon = (6.626 x 10⁻³⁴ J s)(2.998 x 10⁸ m/s) / λ
Solving for λ, we get:
λ = hc/E = (6.626 x 10⁻³⁴ J s)(2.998 x 10^8 m/s) / 9.626 x 10⁻²⁰J/photon
= 2.06 x 10⁻⁷ m
Finally, we convert the wavelength from meters to nanometers:
λ = 2.06 x 10⁻⁷ m x (10⁻⁹ nm/m)
= 206 nm
Therefore, the wavelength of the energy needed to react with silver bromide in 35 mm photographic film is approximately 206 nm.
Find more on photons:
https://brainly.com/question/20912241
#SPJ2
there is no universal rule for the entropy of melting, in contrast to trouton's rule. yet a generic figure is 1kb per particle. assuming this figure is exact, what is the molar enthalpy of melting of argon (in kj/mol), whose melting temperature is 83.85k?
Answers
Entropy is a measurable physical characteristic and a scientific notion that is frequently connected to a condition of disorder, unpredictability, or uncertainty.
Molar enthalpy is the amount of energy per mole. Enthalpy is a thermodynamic quantity that corresponds to a system's total heat content according to this definition. It is equivalent to the system's internal energy plus the sum of the pressure and volume products. This value's unit of measurement is KJ/mol.
Entropy is assumed to be 1 kB/particle
kB: Boltzmann Constant = 1.3806*10^(23)/JK
Entropy, S = 1.3806*10^(-23) /JK
We are aware that S = Q/T
provided T = 83.85 k
and that Q = S*T = S*83.85k = 1.157*10(-21) J / particle.
Since 1 mole = 6.022x1023 particles of heat energy,
the formula for an is 1.157*10(-21)*6.022*10^23, which is 6.967*102 J/mole.
Argon's molar enthalpy is Q/n,
where n is the number of moles,
and ΔH equals 0.6967 KJ/mole.
To know more about Molar enthalpy, click on the link below:
https://brainly.com/question/25758173
#SPJ4
PLEASE HELP !!!
According to the
graph, what happens
to the concentration
of A over time?
(n) uonenu ว
Reaction: 2A A,
Time (sec)
A. It decreases and then levels out.
B. It decreases consistently.
C. It increases and then levels out.
D. It increases consistently.

Answers
According to the graph, the concentration of A decreases with time before leveling out. Option A.
Concentration of a reactant in a reversible reactionThe reaction shown is that of a reversible reaction in which A is on the reactant's side and A2 is on the product's side.
At the beginning of the reaction, the concentration of A decreases as a result of forming A2. In other words, the concentration of A2 increases just as that of A decreases.
With time, the reaction reaches an equilibrium during which the rate of formation of A equals the rate of formation of A2. At this point, the concentration of A levels off.
In summary, the concentration of A first decreases before leveling off.
More on reversible reactions can be found here: https://brainly.com/question/31950205
#SPJ1