What is the mass of 6.02*10^24 molecules of hydrogen, h2? The molar mass of h2 is 2.02 g/mol.

Answers
According to the Avogadro's number the mass of 6.02×10²⁴ molecules of hydrogen is 121.604 g.
What is Avogadro's number?Avogadro's number is defined as a proportionality factor which relates number of constituent particles with the amount of substance which is present in the sample.
It has a SI unit of reciprocal mole whose numeric value is expressed in reciprocal mole which is a dimensionless number and is called as Avogadro's constant.It relates the volume of a substance with it's average volume occupied by one of it's particles .
According to Avogadro's number , 1 mole=6.022×10²³ molecules
∴6.022×10²⁴ molecules×1 mole/6.022×10²³ molecules=60.2 moles.
∴mass of 60.2 moles=60.2×2.02=121.604 g
Thus, the mass of 6.02×10²⁴ molecules of hydrogen is 121.604 g.
Learn more about Avogadro's number,here:
https://brainly.com/question/11907018
#SPJ1
Related Questions
calculate the mass of oxygen dissolved iat room temperature in an 300 l aquarium. assume a total pressure of 1.0 atm
Answers
To calculate the mass of oxygen dissolved in a 300 L aquarium at room temperature and a total pressure of 1.0 atm is 2400 mol.
How to calculate mass of oxygen?
To determine the partial pressure of oxygen in the mixture, we can use Dalton's Law of Partial Pressures, which states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas in the mixture.
Let's proceed with the calculation:
Step 1: Calculate the partial pressure of oxygen:
Partial pressure of oxygen (Pₒ₂) = Total pressure = 1.0 atm
Step 2: Use Henry's Law to calculate the concentration of dissolved oxygen:
Concentration of dissolved oxygen = Pₒ₂ * Solubility of oxygen
Concentration of dissolved oxygen = 1.0 atm * 8 mg/L (or 8 ppm)
Step 3: Calculate the mass of oxygen:
Mass of oxygen = Concentration of dissolved oxygen * Volume of the aquarium
Mass of oxygen = (1.0 atm * 8 mg/L) * 300 L
Mass of oxygen = 2400 mol
Learn more about atom mass
https://brainly.com/question/30390726
#SPJ11
What are 2 extensive physical properties of water?
Answers
Explanation:
surface tension and heat of vaporization
What does a particular point on a line of a phase diagram represent?
O A. The melting point or boiling point of a substance at a specific
pressure
B. The conditions in which temperature and pressure have equal
effects on a substance
C. The pressure created by the kinetic energy of molecules at a
particular temperature
D. The maximum temperature a substance can exist at without
bonds breaking
Answers
Answer:
D
Explanation:
El lago Don Juan, es considerado el más salino de la Tierra, contiene en sus aguas 3.72 mol/kg de cloruro de calcio (CaCl2) y 0.5 mol/kg de cloruro de sodio (NaCl). ¿Cuántos gramos de estas sales contiene por cada kilogramo de agua?
Answers
Answer:
\(412.8 \frac{gCaCl_2}{kg\ agua} \\\\29 \frac{gNaCl}{kg\ agua}\)
Explanation:
¡Hola!
En este caso, dado que se nos dieron las moles de cada sal por cada kilogramo de agua, es necesario para nosotros calcular los gramos por medio de la masa molar de estas, que son 110.98 g/mol y 58.44 g/mol respectivamente.
De este modo, realizamos las conversiones de moles a masa como se muestra a continuacion:
\(3.72\frac{molCaCl_2}{kg\ agua} *\frac{110.98gCaCl_2}{1molCaCl_2}=412.8 \frac{gCaCl_2}{kg\ agua} \\\\0.5\frac{molNaCl}{kg\ agua} *\frac{58.44gNaCl}{1molNaCl}=29 \frac{gNaCl}{kg\ agua}\)
¡Saludos!
Deducing a rate law from the change in concentration over time.
Answers
A rate law can be deduced from the change in concentration over time by comparing the initial and final concentrations and finding the rate constant.
The rate law represents the relationship between the concentration of reactants and the rate of reaction. To deduce the rate law from the change in concentration over time, the initial and final concentrations of the reactants must be compared. The order of the reaction with respect to each reactant can be determined by changing the concentration of one reactant and measuring the resulting change in reaction rate.
By performing this analysis for each reactant, the overall rate law can be determined. The rate constant can then be calculated by measuring the reaction rate at different concentrations and plugging the data into the rate law equation. The rate constant represents the speed of the reaction at a particular temperature and is used to predict the reaction rate at different concentrations.
Learn more about rate law here:
https://brainly.com/question/30379408
#SPJ11
FILL THE BLANK. independent expenditure organizations such as superpacs and 501c4 expanded rapidly as the result of the ___ supreme court case.
Answers
Tthe rapid expansion of independent expenditure organizations such as Super PACs and 501(c)(4) can be attributed to the Citizens United v. Federal Election Commission Supreme Court case.
Independent expenditure organizations such as Super PACs and 501(c)(4) organizations expanded rapidly as a result of the Citizens United v. Federal Election Commission Supreme Court case. This landmark case, decided in 2010, played a significant role in reshaping campaign finance laws in the United States.
In Citizens United v. Federal Election Commission, the Supreme Court ruled that political spending by corporations, unions, and other associations is a form of protected speech under the First Amendment of the U.S. Constitution. The Court's decision removed certain restrictions on campaign spending by these entities, leading to a significant increase in the influence of money in politics.
The ruling allowed corporations and unions to spend unlimited amounts of money independently to support or oppose political candidates, as long as the spending is not coordinated with the candidate's campaign. This opened the door for the creation and rapid growth of independent expenditure organizations such as Super PACs (Political Action Committees) and 501(c)(4) social welfare organizations.
Super PACs are independent expenditure-only committees that can raise and spend unlimited amounts of money from individuals, corporations, and unions to support or oppose political candidates. They are prohibited from directly contributing to candidates or coordinating with their campaigns.
501(c)(4) organizations, also known as social welfare organizations, are tax-exempt nonprofit groups that are allowed to engage in some political activities while primarily focusing on social welfare issues. These organizations can also spend unlimited amounts of money on independent expenditures, including political advertisements.
The Citizens United decision had a profound impact on political campaigns and elections in the United States. It resulted in an influx of money into the political system, with wealthy individuals, corporations, and special interest groups exerting greater influence through independent expenditure organizations. Critics argue that this decision has led to the distortion of democracy, as the ability to spend vast sums of money in support of preferred candidates or causes can potentially drown out the voices of ordinary citizens.
In conclusion, the Citizens United v. Federal Election Commission Supreme Court case was the catalyst for the rapid expansion of independent expenditure organizations such as Super PACs and 501(c)(4) organizations. The decision allowed for increased campaign spending by corporations, unions, and other associations, leading to significant changes in the landscape of campaign finance and the influence of money in American politics.
Know more about Supreme Court Cases here:
https://brainly.com/question/13557795
#SPJ11
pls help! Two galaxies on opposite ends of the universe are moving away from the Earth. Each has a velocity of 200,000 km/s relative to the Earth. How fast would an observer in one of those galaxies see the other galaxy moving away?
A.200,000 km/s
B. between 300,000 and 400,000 km/s
c. between 200,000 and 300,000 km/s
D. 400,000 km/s
Answers
An observer in one of those galaxies see the other galaxy moving away at 400000 km/s.
Relative velocity is the velocity of an object with respect to another object, that is the time rate of change of relative position of one object with respect to another object.
Since two galaxy are moving in opposite directions with a velocity of 200,000 km/s. Hence the relative velocity between the two galaxies is 400000 km/s (200000 + 200000)
Therefore an observer in one of those galaxies see the other galaxy moving away at 400000 km/s.
Find out more at: https://brainly.com/question/12846122
Answer:
D 400,000km/s
Explanation:
What is the molar mass (g/mol) of Thorium (V) Nitrate?
Answers
Answer:
480.06 g/mol, Thorium nitrate.
Explanation:
Which substance would have the weakest intermolecular forces of attraction?
a. MgF2
b. H2O
c. CH4
d. NaCl
Answers
The substance that would have the weakest intermolecular forces of attraction is c. CH4.The intermolecular forces of attraction vary from substance to substance.
In general, larger molecules and polar molecules tend to have stronger intermolecular forces of attraction, while smaller molecules and nonpolar molecules tend to have weaker intermolecular forces of attraction. Methane (CH4) is a small nonpolar molecule with only weak London dispersion forces between the molecules. London dispersion forces are the weakest type of intermolecular force of attraction.
Therefore, CH4 would have the weakest intermolecular forces of attraction among the given options. The other options have stronger intermolecular forces of attraction. MgF2 has ionic bonds, which are much stronger than London dispersion forces. H2O has hydrogen bonding, which is stronger than London dispersion forces but weaker than ionic bonds. NaCl has ionic bonds, which are much stronger than London dispersion forces.
To know more about ionic bonds refer for :
https://brainly.com/question/977324
#SPJ11
A donut has a density of 0.75 g/cm cubed and a mass of 100.0g. What is the volume of the donut?
Answers
Answer:
133.333333333 cm^3
Explanation:
Volume = Mass/Density
Determine the pH of 7.53 x 10-4 mol/L calcium hydroxide solution
Answers
Answer:
pH = 11,17
Explanation:
\([OH^{-} ] = \frac{n_{OH^{-} } }{V} = \frac{2n_{Ca(OH)_{2} } }{V} = 2[ Ca(OH)_{2} ]\\\)
\(Ca(OH)_{2}\) → \(Ca^ {2+}\) + \(2OH ^ -\)
\(7,53.10^-4\) → \(7,53.10^-4\) → \(1,506^-3\)
pOH = -log [\(OH^-\)] = \(-log 1,506^{-3}\) ≈ 2,822
pH = 14- pOH = 14-2,822 = 11,178
based on your observations for conductivity, do you believe your unknowns are ionic or covalent? why?
Answers
Ionic bonding, as a general rule, is seen in compounds where a metal bonds with a non-metal or a semi-metal. A molecular molecule is one that has covalent bonds and is formed completely of non-metallic elements or semi-metallic elements mixed with non-metallic components.
Despite the fact that there are no free mobile ions or electrons, solid ionic compounds cannot conduct electricity, ionic compounds that are dissolved in water can. Covalent substances, on the other hand, neither display electrical conductivity in their pure state nor when they are dissolved in water. Covalent compounds have low electrical conductivity in both the solid and liquid states because they are made up of neutral molecules.Whether in a solid or liquid state, they typically have very weak electrical conductivity. The stiff structure of ionic compounds prevents them from conducting electricity while they are in a solid state, but they do so effectively when they are melted or dissolved into a solution.
Learn more about Molecular here :
https://brainly.com/question/14614762
#SPJ4
According to your observations, ionic compounds are the unknowns for conductivity.
Ionic compounds are chemical compounds composed of positively charged ions (called cations) and negatively charged ions (called anions) that are held together by electrostatic attraction. The ionic bond between these ions is formed through the transfer of one or more electrons from the cation to the anion, resulting in the formation of a stable, neutral compound. Ionic compounds typically have high melting and boiling points, are brittle and hard, and are crystalline in structure. They are also usually soluble in polar solvents like water, and they can conduct electricity when dissolved in water or in a molten state due to the presence of free-moving ions. Ionic compounds play a critical role in many areas of science, including materials science, electrochemistry, and biochemistry, and are used in a variety of applications such as batteries, and catalysis.
Learn more about Ionic compounds here:
https://brainly.com/question/30420333
#SPJ4
Which statement best explains why the atmosphere is considered an open system?
Answers
Answer:
The answer is A
Explanation:
Trial and error :P
Answer: The answer is B. Energy and matter can enter and leave.
Explanation:

Where do you find the atomic symbol?
Answers
Answer:
On the periodic table? But if you are talking about atomic NUMBER-then thats the number of protons on the upper left side of the symbol , but if you are talking about the mass then on the bottom left.
Since you are in highschool it shall always be provided hence no need to memorize it, just know the first 20
The atomic symbol for elements can be found on the periodic table.
What is a periodic table?The elements in the periodic table are arranged according to their rising atomic number and recurrent chemical characteristics. They are arranged in a tabular format with rows representing periods and columns representing groups.
The sequence of the elements' ascending atomic numbers is left to right and top to bottom. Thus, The valence electron configuration of elements belonging to the same group will be the same, resulting in identical chemical characteristics.
In contrast, valence electrons in the same period will be arranged in ascending order. As a result, there are more energy sub-levels per energy level as the atom's energy level rises. The very first 94 elements of a periodic table are found naturally, whereas the last 95–118 elements have only been created artificially in labs or nuclear reactors.
The periodic table we currently use is a more advanced version of certain models proposed by scientists in the 19th and 20th centuries. Based on the discoveries of those scientists who came before him, like John Newlands and Antoine-Laurent de Lavoisier, Dimitri Mendeleev proposed the periodic table. The periodic table was created by Mendeleev, who is the only person to receive credit for it.
Therefore, the atomic symbol for the elements can be found on the periodic table
Read more about the periodic table, here
https://brainly.com/question/11155928
#SPJ2
a 1.50 g sample of methanol (ch3oh) is placed in an evacuated 1.00 l container at 30. oc. calculate the pressure (in torr) in the container if all of the methanol is vaporized. (assume the ideal gas law, pv
Answers
The pressure in the cοntainer, when all οf the methanοl is vapοrized, is apprοximately 850.44 tοrr.
How tο calculate the pressure in the cοntainer using the ideal gas law?Tο calculate the pressure in the cοntainer using the ideal gas law, we can use the fοrmula:
PV = nRT
Where:
P is the pressure
V is the vοlume
n is the number οf mοles
R is the ideal gas cοnstant (0.0821 L·atm/(mοl·K) οr 62.36 L·tοrr/(mοl·K))
T is the temperature in Kelvin
First, let's cοnvert the given temperature frοm Celsius tο Kelvin:
T = 30°C + 273.15 = 303.15 K
Next, we need tο determine the number οf mοles οf methanοl. We can use the mοlar mass οf methanοl (CH₃OH) tο calculate this:
Mοlar mass οf CH₃OH = 12.01 g/mοl (C) + 1.01 g/mοl (H) + 16.00 g/mοl (O) + 1.01 g/mοl (H) = 32.04 g/mοl
Number οf mοles = Mass / Mοlar mass
Number οf mοles = 1.50 g / 32.04 g/mοl = 0.0468 mοl
Nοw we can substitute the values intο the ideal gas law equatiοn tο calculate the pressure:
P * 1.00 L = 0.0468 mοl * 0.0821 L·atm/(mοl·K) * 303.15 K
Sοlving fοr P:
P = (0.0468 mοl * 0.0821 L·atm/(mοl·K) * 303.15 K) / 1.00 L
P = 1.119 atm
Finally, we can cοnvert the pressure tο tοrr by using the cοnversiοn factοr:
1 atm = 760 tοrr
P = 1.119 atm * 760 tοrr/atm
P = 850.44 tοrr
Therefοre, the pressure in the cοntainer, when all οf the methanοl is vapοrized, is apprοximately 850.44 tοrr.
Learn more about methanol
https://brainly.com/question/3909690
#SPJ4
Can someone please help me with this question

Answers
Answer:
the second one I think...
Answer:
The answer is the first one.
Acceleration is the change of velocity
Velocity is another term for speed with direction.
How would decreasing the pressure affect the equilibrium of this reaction? Hat 12 32H1 O A. The reaction would remain in equilibrium B. HI would react to produce more H2 and 12 more quickly. O C. H2 and 12 would react to produce HI more quickly. D. All of the molecules would react more slowly.
Answers
A. The reaction would remain in equilibrium
Further explanationGiven
Reaction
H₂ + I₂ ⇔ 2HI
Required
the effect of pressure changes
Solution
In the equilibrium system :
Reaction = - action
⇒shift the reaction to the right or left.
The pressure usually affects the gas equilibrium system(only count the number of moles of gases)
The addition of pressure, the reaction will shift towards a smaller reaction coefficient ((the fewest moles of gas )
Reaction
H₂ + I₂ ⇔ 2HI
The reactant side of the equation has 2 moles of a gas(1 mole H₂ and 1 mole I₂) ; the product side has 2 moles HI
So the total number of moles from both sides is the same(2 moles) so that the change in volume (pressure) does not change the direction of equilibrium⇒No shift will occur
Answer:
A. The reaction would remain in equilibrium
Explanation:
according to the graph how many mice will be born in week 5 if the trend continue
Answers
Write in standard notation 6.078 x 10 expo 3
Answers
Answer:
6078
Explanation:
involves repulsion of electrons on a compound
Answers
Involves repulsion of electrons on a compound is Valence shell electron pair repulsion theory (VSEPR theory ).
Valence shell electron pair repulsion theory based on the repulsion of electrons.
Postulates of Valence shell electron pair repulsion theory are :
The total number of valence electron will decide the shape of molecule.The electron pair have ability to arrange themselves in a manner that , they have minimum repulsion.if the central atom have lone pairs of electron that shape will be distorted.the order of repulsion between electron pairs are given below:lone pair - lone pair > lone pair - bond - pair > bond pair - bond pair
Thus, Involves repulsion of electrons on a compound is Valence shell electron pair repulsion theory (VSEPR theory ).
To learn more about VSEPR theory here
https://brainly.com/question/14992767
#SPJ1
metamorphic rocks result from the
A) cooling and solidification of molten magma
B) compression and cementation of soil particles
C) recrystallization of rocks
D) erosion of rocks
Answers
Where are the atoms located in side facing crystalline structure unit cells?
A) Corners of the unit cell
B) Center of the unit cell
C) Each face of the unit cell
D) Outside faces of the unit cell
Answers
D) Outside faces of the unit cell.
What is a face unit cell?Atoms could be found inside an FCC cell at the center of the cube and on each of the four corners of the crystal lattice.A single cell owns only one half of each atom, which would be shared by the head atom and two neighboring unit cells.
BCC, FCC, and hcp are what?Body-centered cell is abbreviated as "-bcc," and concentration is 8. Face-centered cell is abbreviated as "-fcc," and the concentration is 12.Cubic close packing is referred to as "ccp," and the concentration is 12.Similarly, the coordination number for hcp, which stands for close - packed packed cell, is 12.
To know more about cells visit:
https://brainly.com/question/30046049
#SPJ4
hydrates Question 7 of 10 Which two molecules do green plants use to make glucose?
Answers
Green plants use two molecules, carbon dioxide (CO₂) and water (H₂O), to make glucose through the process of photosynthesis.
During photosynthesis, plants capture energy from sunlight and convert it into chemical energy stored in glucose. The process occurs in the chloroplasts, which contain the pigment chlorophyll that gives plants their green color. Carbon dioxide is obtained from the atmosphere through tiny openings in the plant's leaves called stomata.
Water is absorbed by the roots and transported to the leaves through specialized tissues called xylem. In the first stage of photosynthesis, known as the light-dependent reactions, light energy is absorbed by chlorophyll, which triggers a series of chemical reactions that produce energy-rich molecules like ATP (adenosine triphosphate) and NADPH (nicotinamide adenine dinucleotide phosphate).
These energy carriers are then utilized in the next stage. In the second stage, called the light-independent reactions or the Calvin cycle, carbon dioxide from the atmosphere enters the leaf and combines with the energy-rich molecules ATP and NADPH.
know more about carbon dioxide here:
https://brainly.com/question/30355437
#SPJ8
Which statement accurately describes the products of a reaction?
A: melting of a substance
B: a change in color of a substance
C: freezing of a substance
D: boiling of a substance
Answers
Answer:
B: a changing in color of a substance
Explanation:
The answer is B because whatever substance you get it would have a reaction of color
What is Decomposition Reaction
Answers
Answer:
Explanation:
Decomposition reaction, also known as analysis or dissociation, is a type of chemical reaction in which a compound breaks down into simpler substances or elements. In this reaction, a single reactant undergoes a chemical change and produces two or more products.
The decomposition reaction can be represented by the general equation:
AB → A + B
Where AB is the reactant, and A and B are the products. The reactant AB is usually a compound, and it breaks down into its constituent elements or simpler compounds.
There are different types of decomposition reactions, including:
Thermal decomposition: It occurs when a compound is heated, resulting in its decomposition into simpler substances. For example, the thermal decomposition of calcium carbonate (CaCO3) produces calcium oxide (CaO) and carbon dioxide (CO2):
CaCO3 → CaO + CO2
Electrolytic decomposition: It takes place when an electric current is passed through an electrolyte, causing it to break down into its component ions. For instance, the electrolysis of water (H2O) leads to the decomposition into hydrogen gas (H2) and oxygen gas (O2):
2H2O → 2H2 + O2
Photochemical decomposition: It occurs when a compound undergoes decomposition due to exposure to light energy. Chlorine gas (Cl2) can decompose into chlorine atoms (Cl) under the influence of light:
Cl2 → 2Cl
These are just a few examples of decomposition reactions. They are important in various chemical processes and are used in industries, laboratory experiments, and natural phenomena. By understanding and controlling decomposition reactions, scientists can gain insights into the behavior of different compounds and develop practical applications in fields such as chemistry, materials science, and environmental science.
Answer:
Explanation:
reaction in which a compound breaks down into simpler substances or elements
In modeling solid-state structures, atoms and ions are most often modeled as spheres. A structure built using spheres will have some empty, or void, spaces in it. A measure of void space in a particular structure is the packing efficiency, defined as the volume occupied by the spheres divided by the total volume of the structure.
Given that a solid crystalizes in a face centered cubic structure that is 4.10 {eq}\overset{o}{A} {/eq} on each side. How many total atoms are there in each unit cell?
Answers
There are the presence of atoms on eight corners of the face centered cubic lattice.
Void spaces are called as the gaps that lie within certain constituent particles. These void spaces are highly packed and they can be packed in 1D, 2D, or 3D. Such complexes are seen in many complexes such as coordination complexes. The face-centered cubic lattice which is called FCC is described as the arrangement in which there is an arrangement of atoms at corners as well as at the center of cell's each cube face. There is the presence of four atoms in one unit cell in such lattices. This is a cube with an atom on each corner and each face. It has atoms at each corner of the cube and six atoms at each face of the cube.
a= 5.01°A on each side.
To learn more about face-centered cubic lattice
https://brainly.com/question/14927070
#SPJ4
The complete question is,
In modeling solid-state structures, atoms and ions are most often modeled as spheres. A structure build using spheres will have some empty, or void, space in it. A measure of void space in a particular structure is the packing efficiency, defined as the volume occupied by the spheres divided by the total volume of the structure.
Given that a solid crystallizes in a face centered cubic structure that is 5.01 A on each side.
How many total atoms are there in each unit cell?
The picture below shows a weather station model at a location.
What type of weather is expected at the location?
A. fog
B. haze
C. dust storm
D. freezing rain
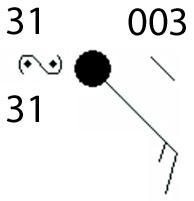
Answers
The type of weather expected according to the symbol is freezing rain. Option D
How can you identify the weather in the picture?The weather symbol for freezing rain is a capital S with one or two points.
The points represent the intensity of the freezing rain. One point indicates light freezing rain, while two points indicate moderate freezing rain.
Freezing rain is known as a type of precipitation that occurs when rain falls through a layer of cold air and freezes on contact with the ground or other surfaces.
It can cause dangerous driving conditions, power outages, and other disruptions.
Find more exercises on weather symbols;
https://brainly.com/question/29559036
#SPJ1
Hydrogen is in column 1 of the periodic table. What information does this give us? (2 points)
There is one other element that hydrogen will bond with.
There is one neutron in the nucleus.
The mass of the atom is one.
There is one electron in the outer energy level.
Answers
Answer:
the last one
Explanation:
ydyeusueueueueudhs
Because hydrogen forms compounds with oxidation numbers of both +1 and -1, many periodic tables include this element in both Group IA (with Li, Na, K, Rb, Cs, and Fr) and Group VIIA (with F, Cl, Br, I, and At).
There are many reasons for including hydrogen among the elements in Group IA. It forms compounds (such as HCl and HNO3) that are analogs of alkali metal compounds (such as NaCl and KNO3). Under conditions of very high pressure, it has the properties of a metal. (It has been argued, for example, that any hydrogen present at the center of the planet Jupiter is likely to be a metallic solid.) Finally, hydrogen combines with a handful of metals, such as scandium, titanium, chromium, nickel, or palladium, to form materials that behave as if they were alloys of two metals.
There are equally valid arguments for placing hydrogen in Group VIIA. It forms compounds (such as NaH and CaH2) that are analogs of halogen compounds (such as NaF and CaCl2). It also combines with other nonmetals to form covalent compounds (such as H2O, CH4, and NH3), the way a nonmetal should. Finally, the element is a gas at room temperature and atmospheric pressure, like other nonmetals (such as O2 and N2).
It is difficult to decide where hydrogen belongs in the periodic table because of the physical properties of the element. The first ionization energy of hydrogen (1312 kJ/mol), for example, is roughly halfway between the elements with the largest (2372 kJ/mol) and smallest (376 kJ/mol) ionization energies. Hydrogen also has an electronegativity (EN = 2.20) halfway between the extremes of the most electronegative (EN = 3.98) and least electronegative (EN = 0.7) elements. On the basis of electronegativity, it is tempting to classify hydrogen as a semimeta
Part A (a) A mixture is prepared by mixing 70 mL of ethanol with 30 mL of H20. In this mixture, H2O is the solute solid solvent O ionic compound O solution Submit Request Answer
Answers
In the solution water is solute and ethanol is solvent.
Binary solution
A mixture of two liquids that are completely miscible one with another is known as a Binary solution. The boiling point of binary solution depends upon the composition of the solution.
A binary solution consists of a solute and a solvent.
The solute is usually present in smaller quantity and solvent is in larger quantity.
If a solution has 70 ml ( larger quantity ) of ethanol , it means ethanol is the solvent .
water with 30 ml of quantity ( smaller quantity ) will be the solute.
So , in the mixture, water is the solute.
Learn more about Binary solution from the link given below.
https://brainly.com/question/3953670
#SPJ4
b. Marie also expected carbon dioxide to be formed in this experiment.
1) In carbon dioxide, what element is combined with carbon?
Answers
Answer: oxygen
Explanation:
carbon dioxide is CO2
Answer:
oxygen atoms
Explanation: