what is the iupac name of this compound? there is a structure of a ch3cch3 backbone, with a –ch3 and an –oh groups attached to the second (from left to right) carbon.
Answers
The IUPAC name of the compound described is 2-methyl-2-propanol.
To determine the IUPAC name, we start by identifying the longest continuous chain of carbon atoms in the molecule, which in this case is a propane backbone (three carbon atoms). Next, we locate the substituents attached to the carbon chain.
In the given compound, there is a methyl group (-CH3) attached to the second carbon atom from the left, and an hydroxyl group (-OH) is also attached to the same carbon atom. The methyl group is named as a substituent, giving the prefix "methyl" to indicate one carbon atom attached to the main chain. The hydroxyl group is named as "ol" to indicate an alcohol functional group.
Combining all the information, the complete IUPAC name for the compound is 2-methyl-2-propanol. The number "2" before "methyl" indicates the position of the substituent on the carbon chain, while "2-propanol" signifies the presence of a three-carbon chain with a hydroxyl group attached to the second carbon.
Know more about IUPAC here:
https://brainly.com/question/16631447
#SPJ11
Related Questions
How many grams are there in 7.40 moles of AgNo3

Answers
Answer: the number of grams of AgNO3 present in its 7.4 moles is 1257.
If a water source has a hundredfold decrease in h concentration from 5.5, what is the new ph of the water source?
Answers
Correct option: If a water source has a hundredfold decrease in H⁺ concentration from 5.5, so the pH will increase by 2 units i.e. 7.5.
What do you mean by pH?The term pH, which originally stood for "potential of hydrogen" (or "power of hydrogen"), is used in chemistry to describe how acidic or basic an aqueous solution is. The pH scale measures the difference between basic and alkaline solutions and acidic solutions (solutions with higher amounts of H+ ions). The pH scale is logarithmic and inversely proportional to the hydrogen ion concentration in the solution. Acidic solutions have a pH under 7, while basic solutions have a pH over 7, at a temperature of 25 °C (77 °F). In this temperature range, neutral solutions have a pH of 7. (e.g. pure water). If the temperature rises above 25 °C, the pH neutral value falls below 7, and vice versa.
To know more about pH, visit:
https://brainly.com/question/15289741
#SPJ4
Ecclesiastes 1:4-11 discusses the consistency and predictability of life. Does the nature of radioactive decay support this or contradict this? Please explain this in detail.
Answers
Answer:
50 Points Help 1) Find the mass of 250.0 mL of benzene. The density of benzene is 0.8765 g/mL.
7) What volume of silver metal will weigh exactly 2500.0 g. The density of silver is 10.5 g/cm3.
Answer:
At 13.6 C, a 1.2 mole sample of a gas exerts a pressure of 2.4 atmospheres. 1 point If you were solving for volume, what number would you use for temperature in the ideal gas law equation (PV = nRT)? о O 13.6 o 1.2 0 24 O 286.6 0 275.4
Thank You and All Answers are found on URL below:
https://answer-helper.com/poisk?searchword=Ecclesiastes+1%3A4-11+discusses+the+consistency+and+predictability+of+life.+Does+the+nature+of+radioactive+decay+support+this+or+contradict+this%3F+Please+explain+this+in+detail.There can be emissions of radiations like gamma radiation. There can be emission of particles too like alpha particle. Therefore, the nature of radioactive decay does not support the given statement.
What is nuclear decay?Nuclear decay is process in which the radioactive element releases particles or radiations. Alpha particles is ⁴₂He. Alpha particle is nothing but helium particle. Decay of radioactive element always comes under first order kinetics.
The rate law for first order kinetics is
K=(2.303/T)×log(a/a-x)
half life=0.693/K
Where
k - rate constant
t - time passed by the sample
a - initial amount of the reactant
a-x - amount left after the decay process
Consistency and predictability are imperatives to the experience of a safe environment. The nature of radioactive decay does not support this.
Therefore, the nature of radioactive decay does not support the given statement.
To know more about nuclear decay, here:
https://brainly.com/question/21114779
#SPJ2
Can someone please help me

Answers
4. Describe what control groups, dependent variables, and
independent variables are. Give an example of each.
Answers
Dependent Variable - a variable whose value depends on that of another
Independent Variable - whose variation does not depend on that of another
Examples:
Height is dependent
Fertilizer is independent
Plants not receiving fertilizer are the control group
A student in the initial stage of expertise in a particular domain often does which of the following?
A. Changes and combines strategies to solve the given problem
B. Experiences beginner's luck when acquiring expert knowledge
C. Experiences difficulty distinguishing between accurate or inaccurate and relevant or irrelevant information
D. Becomes discouraged by failure and attempts to become an expert in a different domain
Answers
A student in the initial stage of expertise in a particular domain often experiences difficulty distinguishing between accurate or inaccurate and relevant or irrelevant information. Option C.
It takes time and practice for a student to develop the necessary skills to identify and prioritize important information in a given domain.
Therefore, they may struggle with determining what information is valuable to solving a problem. However, they may also change and combine strategies to solve the given problem, as they are still exploring different approaches to the domain.
Beginner's luck is not a common experience in acquiring expert knowledge, and becoming discouraged by failure and attempting to switch domains is not an ideal approach to developing expertise.
Hence, the right answer is option C.
Read more about the Domain at https://brainly.com/question/30133157
#SPJ11
The VSEPR model is used mainly to do what
Answers
ASAP...What cause-and-effect relationships are being suggested or predicted based on smaller-scale mechanisms in the systems described by the claims?
Answers
Answer:
Cause and effect relationships are routinely identified, tested, and used to explain change.
Explanation:
If a lab requires each lab group to have 25ml of a solution and it takes 15 grams of CuNO3 to make 1 liter of solution how many grams are needed to make enough solution for 22 lab groups ?
Answers
We need to do some general algebra here.
We will find that you need 8.25 grams of CuNO₃ to make enough solution for the 22 labs.
We know that:
Each lab group needs 25 ml of solution.it takes 15 g of CuNO₃ to make one L of that solution.There are 22 labs.Because each lab needs 25 ml of solution, 22 labs will need that amount 22 times, so the total amount of solution needed is:
22*25ml = 550 ml
Now we know that we need 15 grams to make one liter of solution, and:
1 L = 1000ml
Then you need 15g to make 1000ml
and x (we want to find this amount) to make 550ml
Then we can write two equations (not actual equations, as these are different units) like:
x = 550ml
15g = 1000ml
Now we can take the quotient between these two equations:
x/15 g = (550ml/1000ml)
And now we can solve this for x:
x = (550ml/1000ml)*15g = 8.25g
So you need 8.25 grams of CuNO₃ to make enough solution for the 22 labs.
If you want to learn more, you can read:
https://brainly.com/question/8743486
Can somebody tell me the answers on which one I should circle correctly for each question!!! WILL MARK BRAINLIEST
(Grade7science)
Thanks!

Answers
Answer:
1. False
2. True
3. True
4. True
5. False
Explanation:
28) Correctly record
the volume of this
box.

Answers
Answer:
1653.21 cm
Explanation:
volume formula of rectangular prism is volume times height times width
40.1 times 4.457 times 9.25 = 1653.21
Answer:
The volume of cuboid is 1653.21 cm³
Step-by-step explanation:
GIVEN :
Length of cuboid = 40.1 cmBreadth of cuboid = 4.457 cmHeight of cuboid = 9.25 cmTO FIND :
Volume of cuboidUSING FORMULA :
\(\quad{\star{\underline{\boxed{\sf{V_{(cuboid)} = lbh}}}}}\)
V = volume l = length b = breadth h = heightSOLUTION :
Substituting all the given values in the formula to find the volume of cuboid :
\(\quad{\longrightarrow{\sf{V_{(cuboid)} = lbh}}}\)
\(\quad{\longrightarrow{\sf{V_{(cuboid)} = l \times b \times h}}}\)
\(\quad{\longrightarrow{\sf{V_{(cuboid)} = 40.1 \times 4.457 \times 9.25}}}\)
\(\quad{\longrightarrow{\sf{V_{(cuboid)} = 178.7275 \times 9.25}}}\)
\(\quad{\longrightarrow{\sf{V_{(cuboid)} = 1653.21272}}}\)
\(\quad{\longrightarrow{\sf{V_{(cuboid)} \approx 1653.21}}}\)
\(\quad{\star{\underline{\boxed{\sf{V_{(cuboid)} = 1653.21 \: {cm}^{3}}}}}}\)
Hence, the volume of cuboid is 1653.21 cm³.
————————————————What do the red arrows represent

Answers
Answer:
There are three forces acting on the skateboard. One of these forces is the weight of the rider. Another is the force of gravity on the board itself. The third is the force of the ground pushing up on the skateboard.
What element has 2 valence electrons and 2 energy levels
Answers
Answer:
helium
Explanation:
helium has 2 protons and 2 electrons. The 2 electrons are on the first energy level.
How do the ramp heights of the different objects compare? How does the ramp height relate to the strength of the frictional force between the book and the object?
Answers
The height of a ramp does not directly determine the strength of the frictional force between a book and an object.
How do they compare?The strength of the frictional force between a book and an object is not directly influenced by the height of a ramp. The nature of the surfaces in contact, the force forcing the surfaces together (normal force), and the coefficient of friction are some of the variables that affect the frictional force between two surfaces.
The coefficient of friction between the book and the object plays a major role in determining the strength of the frictional force.
Learn more about frictional force:https://brainly.com/question/30280206
#SPJ1
the amount of fluorine in a metal fluoride is 14.96%. 2 Chromium are connected to the metal when metal chromate is formed. What is the relative atomic mass of the metal
Answers
Answer:
The relative atomic mass of the metal is 207.2 u
Explanation:
Metal chromate
Given that;
1) The mass of fluorine is 14.96% of the metal fluoride
2) 2 Chromium are connected to the metal when the metal chromate, CrO²⁻, is formed
We have;
Number of ions available in the metal = Cr₂O₇²⁻ = +2 ionic
Molar mass of fluorine = 18.998 g/mole
Ionization of fluorine = -1
Number of moles of fluorine required per metal +2 ion= 2 moles
3) Number of moles of fluorine per mole of compound of the metal fluoride = 2 × moles
Mass of fluorine per mole of compound = 2 × 18.998 = 37.996 grams
Percentage by mass of fluorine = 14.96%
Fluoride
Let the mass of the compound = X
Therefore;
14.96% of X = 37.996 grams
X = 37.996/(0.1496) = 253.984 grams
Therefore the mass of the metal in the compound = 253.984 - 37.996 = Molar mass 215.99 grams
Given that the metal forms a chromate with 2 chromium atoms and a mass of 215.99 grams, the likely candidate is lead, Pb with a molar mass of 207.2 grams and a chromate of Pb(CrO₄)₂.
The fluoride, lead fluoride, F₂
The relative atomic mass of lead is 207.2 u
How many significant figures do the following numbers have?
7.
36,004
8.
10.040
9.
0.03460
10.
2.804
11.
4000
12.
13.04 x 104
Answers
Answer:
7. 5
8. 4 or 5
9. 3 or 4
10. 4
11. 1 or 2 or 3 or 4
12. 6
Explanation:
7. 5
8. 4 (excluding the 0) or 5 (including the 0)
9. 3 (excluding the 0 at the back) or 4 (including the 0 at the back)
10. 4
11. 1 or 2 or 3 or 4 (according to the number or 0s included)
12. 13.04 × 104 = 1356.16
ans: 6
8. metallic gold reacts in a 1:1 stoichiometric ratio with metallic cesium to form the salt csau. this material is transparent with mechanical properties similar to that of table salt. the other two group 11 elements, silver and copper do not react with cesium in this manner. a. explain why gold is the only group 11 element with an isolable 1- anion. b. based on the reason you cited for part a, would you expect uranium metal to also have a high enough electrophilicity to form anions? why or why not. c. csau has the same lattice type as csi meaning that the x-ray diffraction pattern of csau has reflections in similar patterns to those of csi, but the diffraction patterns are slightly different. what are differences in the diffraction patterns between these two materials? why?
Answers
A. Gold is the only group 11 element that forms a stable 1- anion because it has the highest electrophilicity among the group 11 elements. Electrophilicity is the measure of an element's ability to attract electrons, and gold has the highest electrophilicity due to its electron configuration and its high electron affinity, which is the energy released when an electron is added to a neutral atom. Gold's high electrophilicity allows it to react with cesium, which is a highly electropositive metal, and form anionic species, such as CSau^-.
B. Based on the reason cited for part A, we would not expect uranium metal to form anions. Uranium has a lower electrophilicity than gold and its electron affinity is also lower, meaning it would be less likely to attract electrons from other elements and form anions.
C. The differences in the diffraction patterns between CSi and CSau are due to the fact that the two materials have different crystal structures. CSi has a sodium chloride-type crystal structure, while CSau has a cesium chloride-type crystal structure. This difference in crystal structure results in slight differences in the spacing between the atoms in the two materials, which leads to differences in the diffraction patterns. The diffraction pattern of a material provides information about its crystal structure, so the slight differences in the diffraction patterns between CSi and CSau are a result of the difference in their crystal structures.
Learn more about electrophilic here: https://brainly.com/question/30026567?referrer=searchResults
#SPJ4
what happens to the speed of a sound wave from an underwater animal as the sound passes into the air above?
A. It stays the same
B. It falls to zero
C. It decreases
D. It increases

Answers
i believe so.
At 298 K, a cell reaction exhibits a standard emf of 0.115 V. The equilibrium constant for the reaction is 6.85 x 105.What is the value of n for the cell reaction?a) 1. b) 2. c) 3. d) 4. e) 5.
Answers
The value of "n" for the cell reaction is approximately 2 (option b). To determine the value of "n" for the cell reaction, we need to consider the relationship between the standard cell potential (E°cell) and the equilibrium constant (K) of the reaction.
The Nernst equation relates the standard cell potential to the equilibrium constant:
E°cell = (0.0592 V/n) * log(K)
Where:
E°cell = Standard cell potential
n = Number of moles of electrons transferred in the balanced equation
K = Equilibrium constant
Given:
E°cell = 0.115 V
K = 6.85 x 10^5
We can rearrange the equation to solve for "n":
n = (0.0592 V) / (E°cell * log(K))
Substituting the given values:
n = (0.0592 V) / (0.115 V * log(6.85 x 10^5))
Using a calculator, we can find:
n ≈ 1.98
Learn more about The Nernst equation: https://brainly.com/question/13043546
#SPJ11
Consider the following oxidation reactions and their equilibrium constants. Rank these metals from strongest to weakest reducing agent. Strongest reducing agent Weakest reducing agent Cu Cd Ni
Answers
Oxidation and reduction are important concepts in chemistry, and they often go hand in hand with redox reactions. Metals that can be oxidized are referred to as reducing agents, whereas those that can be reduced are referred to as oxidizing agents.
The metal that has the greatest tendency to be oxidized is the strongest reducing agent, while the one with the least tendency to be oxidized is the weakest reducing agent. Copper (Cu), cadmium (Cd), and nickel (Ni) are three metals that will be considered. The oxidations and their equilibrium constants are given below:
Cu → Cu2+ + 2 e− [Cu2+] / [Cu]2Cd
→ Cd2+ + 2 e− [Cd2+] / [Cd]2Ni
→ Ni2+ + 2 e− [Ni2+] / [Ni]2
Using the above given equations, equilibrium constants can be determined for these metals:
Cu: Kc = [Cu2+] / [Cu]2
Cd: Kc = [Cd2+] / [Cd]2
Ni: Kc = [Ni2+] / [Ni]2
The smaller the value of Kc, the more spontaneous the reaction. Hence, the metal with the smallest Kc would be the strongest reducing agent, and the metal with the largest Kc would be the weakest reducing agent. According to the above equations, the metals in order of strength from the strongest to the weakest reducing agent are as follows: Cu, Cd, Ni. Therefore, copper is the strongest reducing agent, cadmium is the second strongest reducing agent, and nickel is the weakest reducing agent.
To know more about spontaneous visit-
https://brainly.com/question/5372689
#SPJ11
10 facts about “molecules”
Answers
Answer:
Explanation:
A perfect diamond is a single molecule made of carbon atoms.
DNA is a super long molecule that has information uniquely describing every human being.
When two or more atoms chemically bond together, they form a molecule.
They are very small in size.
They have space between them.
They are in constant random motion.
molecules move when Particles move rapidly in all directions but collide with each other more frequently than in gases due to shorter distances between particles.
In 1926, French physicist Jean Perrin received the Nobel Prize in physics for proving, conclusively, the existence of molecules.
Molecules can exist in free state because they are very stable.
The smallest particle of a compound capable of existing on its own is a Molecule-not a atom.
On the periodic table, why are all noble gases placed in column 18 (8A)?
A.Noble gas elements have identical molar masses.
B.Noble gases are all rarer than elements in other columns.
C.The noble gases only chemically react with other noble gases.
D.The valence electron configuration is similar in all noble gas elements.
Answers
Answer:
D
Explanation:
In the periodic table the noble gases are place in same column because the valence electron configuration is similar in all noble gas elements. Thus option D is correct.
What is periodic table?A periodic table is defined as an organization of all known elements in increasing atomic number and repeating chemical properties.
It can also be defined as a tabular arrangement of chemical elements ordered by increasing atomic number and element groups.
There are more than 118 elements are arranged in modern periodic table.
Noble gases are defined as any of a class of rare gases that includes helium, neon, argon, krypton, xenon, and, most commonly, radon and display high stability and extremely low reaction rates.
Electronic configuration are defined as the electron configuration around the nucleus of a specific atom or molecule.
Thus, in the periodic table the noble gases are place in same column because the valence electron configuration is similar in all noble gas elements. Thus option D is correct.
To learn more about periodic table, refer to the link below:
https://brainly.com/question/11155928
#SPJ5
60 points one of the questions im stuck on
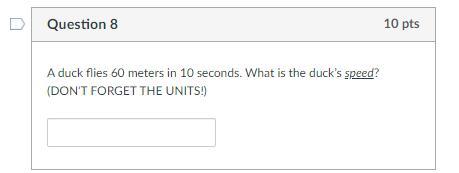
Answers
Answer:
6 m/s is the answer.
Explanation:
speed = distance/time
(please mark me brainliest if you can)
Answer:
6 meters per second
Explanation:
they fly 60 meters in 10 seconds. So to find how many meters they fly in one second, we divide by 10.
60m/10secs = 6 m/s
Pls help
#8: In at least three sentences, describe how the chocolate/wax is like the tectonic plates
on the surface of the Earth. Use the following terms in your answer: tectonic plates,
convection currents, mantle and density. 3 pts
Answers
Explanation:
The chocolate or wax in the activity can be compared to the tectonic plates on the surface of the Earth. Just like the tectonic plates, the chocolate or wax is made up of different layers that move and interact with each other. The movement of the tectonic plates is driven by convection currents in the mantle, which is the layer of the Earth beneath the crust. These convection currents are caused by differences in density between the hot, less dense material near the core of the Earth and the cooler, more dense material near the surface. Similarly, the movement of the chocolate or wax is driven by the heat from the flame, which causes the less dense, melted chocolate or wax to rise and the more dense, solid chocolate or wax to sink.
A high school chemistry teacher asks four student groups to determine the number of protons, neutrons, and electrons (respectively) present in an ion of Titanium (Ti 2+). Which student group provides the best answer?
Answers
Answer:
Electrons - 20
protons - 22
neutrons - 26
Explanation:
Titanium has an atomic number of 22 indicating that the neutral atom has 22 protons.
Ti 2+ has lost two electrons and contains only 20 electrons, 22 protons.
The mass number of titanium is 48. Hence;
Mass number = atomic number + number of neutrons
Since the atomic number is 22 and the mass number is 48
number of neutrons = 48 - 22 = 26 neutrons
how would the yield be affected in ch3cooh(aq) is used in place of glacial acetic acid in this experiment?
Answers
If CH\(_{3}\)COOH(aq) is used in place of glacial acetic acid in an experiment, the yield will be affected by being reduced due to the difference in concentration and purity of the two solutions.
Glacial acetic acid is a highly concentrated and pure form of acetic acid, while CH\(_{3}\)COOH(aq) is a diluted solution of acetic acid in water. This may lead to a lower yield of the desired product as the reaction may not proceed as efficiently. Additionally, the impurities present in CH\(_{3}\)COOH(aq) may also interfere with the reaction, further reducing the yield. Therefore, it is important to use the correct reagents and ensure that they are of high purity to obtain accurate and reliable results in the experiment.
More on acetic acid: https://brainly.com/question/24078194
#SPJ11
Suppose that money is deposited daily into a savings account at an annual rate of $20,000. If the account pays 5% interest compounded continuously, estimate the balance in the account at the end of 6 years. CAS The approximate balance in the account is 5 (Round to the nearest dollar as needed)
Answers
The approximate balance in the account at the end of 6 years is $159,074. Rounded to the nearest dollar, it is $159,074.
Assuming that the annual rate of $20,000 is deposited at the beginning of each year, the total amount deposited over 6 years would be $120,000. With continuous compounding at 5% interest rate, the formula to calculate the balance in the account after 6 years is:
A = Pe^(rt)
Where A is the balance, P is the principal (amount deposited), e is the mathematical constant approximately equal to 2.71828, r is the interest rate in decimal form, and t is the time in years.
Plugging in the values, we get:
A = $120,000e^(0.05*6)
A = $159,073.51
Therefore, the approximate balance in the account at the end of 6 years is $159,074. Rounded to the nearest dollar, it is $159,074.
To know more about account visit:
https://brainly.com/question/30977839
#SPJ11
Ice at 0.0°C is mixed with 7.30 × 10^2 mL of water at 25.0°C. How much ice must melt to lower the water temperature to 0.0°C? The specific heat capacity of water is 4.186 J/(g·K). Latent heat of fusion for water is 333.7 J/g.
Answers
Approximately 35.90 grams of ice must melt to lower the water temperature to 0.0°C.
To solve this problem, we need to calculate the amount of heat that needs to be transferred from the water to the ice in order to lower the water temperature to 0.0°C.
First, let's calculate the initial heat content of the water. The specific heat capacity of water is 4.186 J/(g·K), and the mass of the water can be calculated using its density (1 g/mL) and volume (7.30 × 10^2 mL):
Mass of water = density × volume = 1 g/mL × 7.30 × 10^2 mL = 7.30 × 10^2 g
The initial heat content of the water can be calculated using the formula:
Heat content = mass × specific heat capacity × temperature change
Heat content = 7.30 × 10^2 g × 4.186 J/(g·K) × (25.0°C - 0.0°C) = 7.30 × 10^2 g × 4.186 J/(g·K) × 25.0°C
Next, we need to calculate the amount of heat that needs to be transferred from the water to the ice to lower the water temperature to 0.0°C. This heat transfer occurs during the melting of the ice.
The amount of heat required to melt the ice can be calculated using the formula:
Heat = mass of ice melted × latent heat of fusion
Let's assume that x grams of ice melts. The mass of the ice can be calculated using its density (0.92 g/mL) and volume (same as the volume of water):
Mass of ice = density × volume = 0.92 g/mL × 7.30 × 10^2 mL = 6.716 × 10^2 g
Heat = x g × 333.7 J/g
Now, we need to ensure that the heat transferred from the water to the ice is enough to lower the water temperature to 0.0°C. The heat transferred from the water to the ice is equal to the heat transferred from the water when its temperature drops to 0.0°C:
Heat content of water = Heat transferred to ice
7.30 × 10^2 g × 4.186 J/(g·K) × 25.0°C = x g × 333.7 J/g
Now, we can solve for x:
x = (7.30 × 10^2 g × 4.186 J/(g·K) × 25.0°C) / (333.7 J/g)
x ≈ 35.90 g
Therefore, approximately 35.90 grams of ice must melt to lower the water temperature to 0.0°C.
Learn more about temperature
https://brainly.com/question/27944554
#SPJ11
carbon paired electrons
Answers
Answer:
what about carbon paired electrons? what do you need to know about them?
Explanation:
Helppp pleaseee
How many hydrogen atoms are indicated by the formula (NH4)2C7H10O2?
Answers
Answer:
The correct answer is - 18.
Explanation:
The number of hydrogen atoms is indicated by the formula (NH4)2C7H10O2 that has two parts (NH4)2 and C7H10O2 then
Number of hydrogen atoms in (NH4)2 = 4*2= 8
number of H atoms in C7H10O2 = 10
then the total number of atoms = 10+8
= 18.
Thus, the correct answer is = 18 hydrogen atoms.