what is the initial molarity if .600 gram of the acid was initially added to 100ml of the water
Answers
Given that 0.600 gram of the acid was initially added to 100 ml of water. We are to determine the initial molarity.Main Answer:Initial molarity of the acid is 0.126 M.:
We know that,Molarity (M) = Number of moles of solute / Volume of solution (in liters)Now, we can calculate the number of moles of the acid using its mass and molecular weight.Number of moles of acid = Mass of acid / Molecular weight of acidMolecular weight of acid = 96 g/mol (Given)Mass of acid = 0.600 gNumber of moles of acid = 0.600 g / 96 g/mol= 0.00625 molThe volume of solution in liters is given as 100 ml = 0.1 L'
.Now, we can calculate the initial molarity of the acid using the formula mentioned above.Molarity of acid = Number of moles of acid / Volume of solutionMolarity of acid = 0.00625 mol / 0.1 L= 0.0625 L= 0.126 MTherefore, the initial molarity of the acid is 0.126 M.
To know more about acid visit:
https://brainly.com/question/29796621
#SPJ11
Related Questions
What are the physical states and chemical forms of matter?
Answers
Answer:
The three states of matter are the three distinct physical forms that matter can take in most environments: solid, liquid, and gas. In extreme environments, other states may be present, such as plasma, Bose-Einstein condensates, and neutron stars.
Explanation:
A 0.100 M oxalic acid, HO2CCO2H, solution is titrated with 0.100 M KOH. Calculate the pH when 25.00 mL of oxalic acid solution is titrated with 35.00 mL of NaOH. Ka1 = 5.4 × 10−2 and Ka2 = 5.42 × 10−5 for oxalic acid
Answers
A 0.100 M oxalic acid, HO₂CCO₂H, solution is titrated with 0.100 M KOH. Calculate the pH when 25.00 mL of oxalic acid solution is titrated with 35.00 mL of NaOH. Ka1 = 5.4 × 10−2 and Ka2 = 5.42 × 10−5 for oxalic acid is 1.94.
Oxalic acid is a dicarboxylic acid with the chemical formula HO₂CCO₂H, with a pKa1 of 1.25 and a pKa2 of 4.14. A titration is a process in which a solution of known concentration is added to a solution of unknown concentration until the equivalence point is reached.
The goal of a titration is to determine the concentration of the unknown solution. A 0.100 M oxalic acid solution is titrated with 0.100 M KOH. The pH is calculated when 25.00 mL of oxalic acid solution is titrated with 35.00 mL of NaOH. Ka1 = 5.4 × 10−2 and Ka2 = 5.42 × 10−5 for oxalic acid. Here is how to calculate the pH when 25.00 mL of oxalic acid solution is titrated with 35.00 mL of NaOH:
Step 1: Calculate the moles of oxalic acid
moles of oxalic acid = concentration x volume
moles of oxalic acid = 0.100 M x 0.025 L
moles of oxalic acid = 0.0025 mol
Step 2: Calculate the moles of NaOH
moles of NaOH = concentration x volume
moles of NaOH = 0.100 M x 0.035 L
moles of NaOH = 0.0035 mol
Step 3: Determine which species is in excess
0.0025 mol of oxalic acid reacts with 0.0025 mol of KOH
0.0010 mol of oxalic acid reacts with 0.0035 mol of KOH
0.0010 mol of oxalic acid is consumed in the reaction
The excess moles of NaOH = 0.0035 - 0.0010 = 0.0025 mol
Step 4: Calculate the concentration of the remaining OH- ions
Concentration of OH- ions = moles of OH- ions / volume
Concentration of OH- ions = 0.0025 mol / 0.060 L
Concentration of OH- ions = 0.0417 M
Step 5: Calculate the concentration of H+ ions
Ka1 = [H+][C₂O₄₂-]/[HC₂O₄-]
5.4 x 10^-2 = x^2 / (0.100 - x)
Assuming that x is much smaller than 0.100, x = [H+] = 0.115 M
Step 6: Calculate the pH
pH = -log[H+]
pH = -log(0.115)
pH = 1.94
To know more about oxalic acid refer here: https://brainly.com/question/9939766#
#SPJ11
Glucose can be found in foods like honey. What happens to glucose in the body?
Answers
Answer: Unused glucose is stored mainly in the liver as glycogen.
Explanation: The body breaks down most carbohydrates from the foods we eat and converts them to a type of sugar called glucose. Glucose is the main source of fuel for our cells. When the body doesn't need to use glucose for energy, it stores it in the liver and muscles. This stored form of glucose is made up of many connected glucose molecules and is called glycogen. When the body needs a quick boost of energy or when the body isn't getting glucose from food, glycogen is broken down to release glucose into the bloodstream to be used as fuel for the cells.
When the body doesn't need to use glucose for energy, it stores it in the liver and muscles.
Need help ASAP . Anybody ?
- 20 points

Answers
Which of the following is not a function of the cell?
Sleeping
Protection
All of the above
Reproduction
Answers
How would the system change if the container were placed on a hot plate? some kinetic energy would be produced. Some kinetic energy would be destroyed. The kinetic energy of the molecules would increase. The kinetic energy of the molecules would decrease.
Answers
If a container is placed on a hot plate, the kinetic energy of molecules inside the container will increase.
It is a fundamental theory of thermodynamics that energy cannot be produced and neither be destroyed. So obviously from the fundamental theorem of thermodynamics, the first two options in the given question ( some kinetic energy would be produced and some kinetic energy would be destroyed ) are obviously wrong.
Since the container is placed on a hot plate, the heat energy will get transferred from the plate to the container, and hence the molecules inside the container will get heated and excited. Therefore the kinetic energy of molecules inside the container will increase.
So basically, in this case, the heat energy of the plate is converted to the kinetic energy of molecules inside the container.
To study more about energy conversion, please refer to the following link - https://brainly.com/question/961052
whats the best animal to have as a pet
Answers
These animals make the best pets for home are dogs,rabbit, cats,fish and tortoises.
What is animals?Animals can be defined as multicellular eukaryotic organisms that form the biological kingdom Animalia. Animals consume organic material breathe oxygen are able to move.
Therefore, some important of domesticated animals are dogs, cats, sheep and goats. Fishes are reared and used for food, fish oil, manure, glue, and some important medicinal purposes and also camels, horses and elephants are used for transportation
Learn more about animals here: brainly.com/question/19884444
#SPJ1
19) While watching the news one night, you see a commercial for a new vehicle that will be powered
by water. This will be accomplished by passing electricity through water, producing pure oxygen
and pure hydrogen. These two gases will then be mixed together and ignited. This process of
passing electricity through a substance to separate molecules is called
A) electrochemistry
C) electrolysis
B) electrorefining
D) electroplating
Answers
The process of passing electricity through a substance to separate molecules is called electrolysis. Option C is correct.
Electrolysis is a process in which an electric current is passed through a conducting medium (such as a liquid or a molten electrolyte) in order to bring about a chemical change. It involves the use of electrical energy to drive a non-spontaneous redox reaction, causing the decomposition or transformation of substances at the electrodes.
Electrolysis has a wide range of applications in various industries. For example, it is used in the extraction of metals from their ores, such as the production of aluminum from bauxite, or the extraction of copper from copper ores.
Electrolysis plays a crucial role in many technological advancements and industrial processes, making it an important area of study in the field of electrochemistry. It has widespread applications in fields such as metallurgy, chemical industry, energy production, and environmental remediation.
Hence, C. is the correct option.
To know more about Electrolysis here
https://brainly.com/question/12994141
#SPJ1
Explain why the following are made of thermosetting plastics.
(a) Saucepan handles
(b) Electric plugs/switches/plug boards
Answers
Answer:
a: they're used in saucepan handles 'cause they don't soften when heated and also 'cause they cant be bent easily.
b: thermosetting plastics are bad electricity conductors. they don't get moulded and are also hard and strong.
how can millimoles (or mmol) be calculated from molarity and volume? given that molarity is defined as mol/l, why can molarity also be calculated from mmol/ml?
Answers
Millimoles (mmol) can be calculated from molarity and volume using the following formula: mmol = M x V, where M is the molarity in mol/L and V is the volume in L. To calculate the molarity from mmol/mL, one can simply divide the mmol value by the volume in mL and convert to mol/L by multiplying by 1000.
Molarity is a measure of the concentration of a solution, defined as the number of moles of solute per liter of solution. Millimoles (mmol) is a unit of measurement that is often used in biochemistry and other fields to express small amounts of substances. To convert between molarity and millimoles, one can use the formula mmol = M x V, where M is the molarity in mol/L and V is the volume in L.
This formula allows one to calculate the number of millimoles of solute present in a given volume of solution, given the molarity. Conversely, to calculate the molarity from millimoles, one can simply divide the mmol value by the volume in mL and convert to mol/L by multiplying by 1000. It is important to use the correct units when making these conversions to ensure accurate results.
To know more about molarity, refer here:
https://brainly.com/question/16727614#
#SPJ11
Adding the reactants together is known as * (1 point) - Product - Compound - Precipitate - Reaction mixture Answer please. I really need it.
Answers
Answer:
Reaction Mixture
Explanation:
Using process of elimination;
Product
This is the substance formed as a result of a chemical reaction. Incorrect option
Compound
A substance formed when tow or more elements are chemically bonded together. Incorrect option.
Precipitate
Name given to an insoluble solid formed during a reaction. Incorrect option.
Reaction Mixture
Process of combing two or more substances to form a reaction. Correct option.
Question (6)-(20\%): Choose the right answer. A concrete weir with a steel sheet pile, 6 m deep, is built at a site where the soil consists of sand strata of depth 16 m. The length of the base of the weir is 20 m, founded at 2 m below ground surface. The weir is to retain 8 m of water in the up stream. The soil has a 4.6
∗
10
−4
cm/sec coefficient of nermeability, specific gravity of 2.65, and void ratio of 0.72. Calculate the
Answers
The height of the sheet pile above the ground surface will be 6 + 5.125 = 11.125 m.Therefore, the correct answer is C, 4.872 m.
The right answer is option C, 4.872 m.Explanation:Given,Depth of weir (h)
= 6 m Soil strata depth
= 16 mBase length of weir (B)
= 20 m Foundation depth (df)
= 2 mHead of water retained (H)
= 8 m Permeability coefficient (k)
= 4.6 × 10⁻⁴ m/s Specific gravity (G)
= 2.65Void ratio (e)
= 0.72
To calculate: The height of sheet pile above the weir crest, h1 Assuming the flow is in the horizontal direction from left to right, the weir will act as a vertical wall. Let h1 be the height of the sheet pile above the weir crest.As the water level will be retained to a height of 8 m, the total head on the soil below the sheet pile will be H + h1. Applying the Darcy's law, we can obtain the seepage velocity as:v
= ki.e., v
= 4.6 × 10⁻⁴ × (8 + h1)
So,The seepage velocity at the weir base will be:v
= 4.6 × 10⁻⁴ × (8 + h1)
= (kh/B) × i.e., 4.6 × 10⁻⁴ × (8 + h1)
= (4.6 × 10⁻⁴ × 16) / 20
Hence,
8 + h1
= 2.875
Solving this equation, we get,h1
= 2.875 − 8
= −5.125 m.
Thus, the height of the sheet pile above the weir crest is -5.125 m which means that the sheet pile will extend 5.125 m below the ground surface. The height of the sheet pile above the ground surface will be
6 + 5.125
= 11.125 m.
Therefore, the correct answer is C, 4.872 m.
To know more about ground visit:
https://brainly.com/question/14795229
#SPJ11
Finally we can calculate the final volume of the solutioWhat final volume will produce a solution in which [Cl–] = 0.233 M, using 3.61 g of CoCl3?
_______ L solutionn.
Answers
The final volume of solution in which [Cl–] = 0.233 M, using 3.61 g of CoCl3 219 L solution.
Thus, M, or moles/liter, is the sign for molarity. Additionally, chemists utilize square brackets to denote a reference to a substance's molarity. The molarity of the silver ion in solution, for instance, is shown by the formula [Ag+].
The most straightforward to calculate with but the most challenging to create in the lab are solution concentrations stated in molarity. When addressing chemical reactions in which a solute is a product or a reactant, such concentration units are helpful.
Then, in order to translate numbers in moles to amounts in grams, molar mass can be employed as a conversion factor. The terms "mol" and "L" in this statement stand for moles of solute.
Thus, The final volume of solution in which [Cl–] = 0.233 M, using 3.61 g of CoCl3 219 L solution.
Learn more about Volume, refer to the link:
https://brainly.com/question/28058531
#SPJ4
Describe the pattern of the Lewis dot structures of the first 18 elements (include periods and groups/families)?
Answers
Answer:
The Lewis dot structures of elements in the same group are the same (except that the name of the element is shown in the structure) while for elements in the same period it increases by one from left to right.
Explanation:
Lewis dot structures are the structures which represent the valence electrons of an atom. The dots in the structure represent electrons in the valence shell of the atom.
Elements in the periodic table are arranged into groups and periods. The groups are the vertical columns whereas the periods are the horizontal rows. The first 18 elements of the periodic table are Hydrogen(H), Helium (He), Lithium (Li), Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F), Neon(Ne), Sodium (Na), Magnesium (Mg), Aluminum (Al), Silicon (Si), Phosphorous (P), Sulfur (S), Chlorine (Cl) and Argon (Ar). They are found within Groups IA to VIIIA, as well as from period 1 to 3 in the periodic table. elements in group IA have one valence electron; elements in group IIA has two, IIIA has three, IVA has four, VA has five, VIIA has six, VIIA has seven, while VIIIA has eight valence electrons.
Elements in the same group have the same number of valence electrons while the valence electrons of elements in the same period increase by one from left to right. Therefore, the Lewis dot structures of elements in the same group are the same (except that the name of the element is shown in the structure) while for elements in the same period it increases by one from left to right.
The Lewis dot structure of the first 18 elements is shown in the attachment below.

Which measure of a gas does the expression
nRT/p
represent? (1 point)
O molar mass
O volume
O number of moles
O gas constant
Answers
Answer:
A. volume
Explanation:
Generally the equation for the ideal gas is mathematically given as
PV=nRT
Where
P=pressure
V=volume
R=gas constant
n=Number of Moles
T=Temperature
Therefore
V=nRT/P
Option A
For more information on this visit
brainly.com/question/17756498
in an ideal solution of a strong electrolyte, the van't hoff factor i is equal to _____.
Answers
"the number of ions produced by one formula unit of the electrolyte," refers to the van't Hoff factor (i) in an ideal solution of a strong electrolyte. It represents the extent of dissociation of the electrolyte into ions.
In an ideal solution of a strong electrolyte, the van't Hoff factor (i) represents the number of ions that are produced when one formula unit of the electrolyte dissociates completely in the solution. It is a measure of the extent of dissociation of the electrolyte.
For example, for a strong electrolyte such as sodium chloride (NaCl), when it dissolves in water, it completely dissociates into sodium ions (Na+) and chloride ions (Cl-). In this case, the van't Hoff factor (i) would be 2 because one formula unit of NaCl produces two ions (Na+ and Cl-).
Similarly, for other strong electrolytes, the van't Hoff factor (i) can be determined based on the number of ions produced per formula unit. It is important to note that for non-electrolytes or weak electrolytes, the van't Hoff factor (i) is typically less than 1, indicating partial dissociation or no dissociation in the solution.
learn more about ions here:
https://brainly.com/question/30663970
#SPJ11
If 1.0 mole of methane reacts with oxygen to produce carbon dioxide and water, what mass of water is produced?
44 grams
16 grams
36 grams
18 grams
Answers
Answer:
36 grams
Explanation:
Predict the equilibrium shift that will occur in the reaction below when the temperature is decreased. N2(g)+O2(g) ➡️⬅️ 2NO(g) H°= 181 kJShifts the reaction towards the reactants Decreasing temperature has no effect on the equilibrium of the reactionMore info is neededShifts the reactants towards the products
Answers
Answer
Shifts the reaction towards the reactants
Explanation
The reaction is endothermic since DH = + so we will take heat as the reactant.
So if temperature is decreased the there will be less heat, this will shift to the left to relieve stress of the reactants
You have 3 moles of Mn3(PO.)4. How many grams are present?
Answers
In this question, we have to find the mass of Mn3(PO4)4, we will be using the molar mass of the compound, which is 544.7g/mol
544.7g = 1 mol
x grams = 3 moles
x = 1634 grams of Mn3(PO4)4
Consider the picture of a gas pump. Which type of gasoline has the highest percentage of octane (the main component of gasoline)?
unleaded: 87 octane rating
mid-grade: 89 octane rating
premium: 91 octane rating
Answers
Answer:
premium: 91 octane rating
Explanation:
Octane number refers to the percentage or volume fraction of isooctane in a fuel.
The octane number gives a picture of how safe a fuel is for an engine. The higher the octane rating the lesser the tendency of the fuel to cause knocking of the engine.
The type of gasoline with the highest percentage of octane among the options is premium.
Methane is the main component of natural gas. Using the given reaction enthalpy, calculate the heat energy produced by the combustion of one kilogram of methane.
Answers
Answer: The heat energy produced is 53831.25KJ
Explanation:
METHANE is the main component of natural gas. It can undergo combustion reaction in air with a bright blue flame to produce carbondioxide and water. The heat of reaction (enthalpy) is negative because heat is absorbed during the chemical reaction. To calculate the heat energy produced by the combustion of one kilogram (1 kg) of methane the following steps are taken:
Molecular mass of methane =16 gm/mol.
So moles of 1 kg methane =
Given mass of methane ÷ molecular weight of methane
But the given mass = 1kg = 1000g
Therefore,
moles of 1000g methane = 1000÷16
= 62.5 moles
Hence, energy evolved = (moles of methane) × (heat of combustion)
Therefore,
heat energy produced= 62.5 × (-861.3kj)
= -53831.25kj

The heat energy produced by the combustion of one kilogram of methane is –53831.25 KJ
We'll begin by calculating the number of mole in 1 Kg of CH₄
Mass of CH₄ = 1 kg = 1000 g
Molar mass of CH₄ = 12 + (4×1) = 16 g/mol
Mole of CH₄ =?Mole = mass /molar mass
Mole of CH₄ = 1000 / 16
Mole = 62.5 moles Finally, we shall determine the heat energy produced from the reaction.CH₄ + 2O₂ —> CO₂ + 2H₂O ΔH = –861.3 KJ
From the balanced equation above,
1 mole of CH₄ reacted to produce –861.3 KJ.
Therefore,
62.5 moles of CH₄ will react to produce = 62.5 × –861.3 = –53831.25 KJ
Thus, –53831.25 KJ of heat energy is produced.
Complete Question is located in the attached photo
Learn more: https://brainly.com/question/13671340

Explain why hydrogen is a substance.
Answers
Answer:
Hydrogen is a substance because it has mass and combined with other molecules it can make another substance such as water.
Explanation:
Rank the following molecules (1st, 2nd, 3rd, 4th) in the order that they melt.
Sucrose
Iodine
Sodium
Paraffin
There is a picture attached

Answers
Sodium chloride has the highest melting point while iodine has the least melting point.
The melting points of solid substances depends on the nature of intermolecular forces that exists in the substance. The stronger the magnitude of intermolecular forces in a substance, the higher its melting point.
The order of melting points of the solids shown in question is as follows;
1st - Sodium chloride2nd - sucrose3rd - paraffin4th - IodineSodium chloride has the highest melting points because it is an ionic substance. Among the molecular sucrose and paraffin, sucrose has a higher melting point than sucrose because it has a greater molar mass. Iodine, a molecular substance has the least molar mass and the least melting point in the list.
Learn more: https://brainly.com/question/25777663
What is the specific volume of water and gas confined in a close vessel if the dryness fraction is 0.75? Assume vf=0.001061 and vg=0.885. a. 0.885 b. 0.664 C. 0.443 d. 0.123
Answers
The specific volume of a substance is defined as the volume occupied by a unit mass of the substance. the specific volume of the water and gas mixture confined in the closed vessel with a dryness fraction of 0.75 is approximately 0.664 m³/kg.
In this case, we need to calculate the specific volume of water and gas confined in a closed vessel with a dryness fraction of 0.75.
Dryness fraction (x) = 0.75
Specific volume of saturated liquid water (vf) = 0.001061 m³/kg
Specific volume of saturated vapor gas (vg) = 0.885 m³/kg
To calculate the specific volume, we can use the following formula:
Specific volume = (1 - x) * vf + x * vg
Substituting the given values:
Specific volume = (1 - 0.75) * 0.001061 m³/kg + 0.75 * 0.885 m³/kg
= 0.25 * 0.001061 m³/kg + 0.75 * 0.885 m³/kg
= 0.00026525 m³/kg + 0.66375 m³/kg
≈ 0.664 m³/kg
Therefore, the specific volume of the water and gas mixture confined in the closed vessel with a dryness fraction of 0.75 is approximately 0.664 m³/kg.
The correct option is (b) 0.664.
To know more about volume:
https://brainly.com/question/28058531
#SPJ4
Question 2
1 pts
What is the molar mass of Cr2(SO4)3?
O 148.1 g
0 288.0 g
O 344.2 g
0 392.2 g
200.0 g
Answers
Answer:
392g
Explanation:
The given compound is;
Cr₂(SO₄)₃
To find the molar mass, we sum the atomic masses of the elements in the compounds together.
Atomic mass of Cr = 52
S = 32
O = 16
So;
Molar mass = 2(52) + 3[32 + 4(16)] = 392g/mol
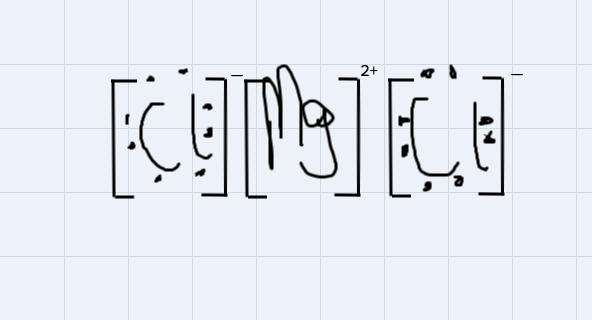
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.
Why do the planets in our solar system orbit in approximately the same plane around the sun?
Answers
http://curious.astro.cornell.edu/about-us/57-our-solar-system/planets-and-dwarf-planets/orbits/242-why-do-all-the-planets-orbit-in-the-same-plane-intermediate
The least abundant gas in the air is
a. Xenon, Xe
b. Oxygen, O2
c. Water, H2O
d. Nitrogen, N2
Answers
Answer:
A.
Explanation:
Two or more than two atoms with different physical or chemical properties can not combine together to form an element. Xenon(Xe ) is the least abundant element on the earth. The correct option is option A.
What is element?Element generally consist of atoms or we can atoms combine to form element. Atoms of an element is always same, means all the properties of all atoms of one type of element is same.
Oxygen, water, and nitrogen are the most abundant molecule on the earth. Xenon is the least abundant amongst all given option. Xenon belongs to noble gas family. So, Xenon is not reactive at all. Xenon does not form any compound with any other element so its abundance on earth is very less.
Therefore, Xenon(Xe ) is the least abundant element on the earth. The correct option is option A.
To know more about element, here:
https://brainly.com/question/8460633
#SPJ2
How do different chemical reactions interact with the surrounding environment?
Answers
Answer:
The earth is made of chemical
Explanation:
Explanation:
because of the chemical made up with earth
Where is earths air the thinnest
the troposphere
the mesosphere
the stratosphere
the thermosphere
Answers
Answer:
Troposphere
Explanation:
the lowest layer of the earths atmosphere