What is the importance of the presence of silicate in and asteroids?
Answers
The presence of silicate minerals in asteroids is significant because they help us understand the composition and evolution of the Solar System. Scientists study asteroids because they believe that they are some of the most primitive and relatively unchanged bodies in the Solar System.
The study of silicate minerals found in asteroids provides us with an idea of the distribution of minerals in the early Solar System. Silicate minerals are the most abundant minerals in the Solar System, making them critical to the study of the early Solar System. The data obtained from these studies helps scientists to understand the formation of planets and the origin of the Solar System, giving us a better understanding of the history of the universe.
The presence of silicate minerals in asteroids also provides us with a source of raw materials for scientific research and commercial purposes. These minerals can be mined and used to study the chemical and physical properties of the Solar System. Additionally, they can be used for the development of new technologies and space exploration. For example, asteroids could be used as a source of water, which is critical for long-duration space missions. In conclusion, the presence of silicate minerals in asteroids is essential to our understanding of the formation and evolution of the Solar System. Their study provides us with valuable data that helps us understand the history of the universe, and they offer us a source of raw materials for scientific and commercial purposes.
To know more about minerals visit:
https://brainly.com/question/29970865
#SPJ11
Related Questions
Calculate the pH of a buffered solution of 0.42 M acetic acid and 0.15 M sodium acetate before AND after 0.0030 moles of NaOH (aq) are added to 0.100 L of the buffer.
Answers
The pH of the buffered solution of 0.42 M acetic acid and 0.15 M sodium acetate before and after 0.0030 moles of NaOH (aq) are added to 0.100 L of the buffer is 4.43 and 4.72, respectively.
A buffer solution is an aqueous solution that resists changes in pH when small amounts of an acid or a base are added to it. A buffer solution is typically made up of a weak acid and its conjugate base, or a weak base and its conjugate acid. The Henderson-Hasselbalch equation, which is used to calculate the pH of a buffer solution, is as follows:pH = pKa + log [A-]/[HA]Where pH is the pH of the buffer solution, pKa is the acid dissociation constant of the weak acid, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid.Before any acid or base is added, the pH of the buffer solution can be calculated as follows:
Acetic acid (CH3COOH) is a weak acid with a pKa of 4.76. Sodium acetate (CH3COONa) is the conjugate base of acetic acid. Therefore, [A-] = 0.15 M and [HA] = 0.42 M - 0.0030 M = 0.417 M. pH = 4.76 + log (0.15/0.417) = 4.43
After 0.0030 moles of NaOH are added to the buffer solution, they will react with the acetic acid to form sodium acetate and water:
CH3COOH (aq) + NaOH (aq) → CH3COONa (aq) + H2O (l)
The amount of acetic acid that reacts with the NaOH is given by the stoichiometry of the reaction:
0.0030 moles of NaOH react with 0.0030 moles of CH3COOH.
Therefore, the new concentration of CH3COOH is 0.417 M - 0.0030 M = 0.414 M.
The new concentration of CH3COONa is 0.15 M + 0.0030 M = 0.153 M.Using these new concentrations, we can calculate the new pH of the buffer solution:
pH = 4.76 + log (0.153/0.414) = 4.72
Therefore, the pH of the buffered solution of 0.42 M acetic acid and 0.15 M sodium acetate before and after 0.0030 moles of NaOH (aq) are added to 0.100 L of the buffer is 4.43 and 4.72, respectively.
To know more about buffered solution visit:
https://brainly.com/question/31367305
#SPJ11
Fill in the blank
Potential energy is energy an object has due to its ____
or condition
Answers
Answer :position
Explanation:
Potential energy is the energy stored in an object due to its position
Pa help po thanks! I need the answer ASAP

Answers
Answer:
1.T
2.I
Explanation:
hope it help
#carry on learning
The object and description that matches is Object 2 and T.
Object 1 has no matching description.
Fish Aquarium FilterA Fish aquarium filter is a filter whose function is to clean the water of debris, removes the toxic buildup of ammonia and nitrates, and aerates the water so that fish can in a conducive environment and breathe properly
Engine Oil FilterAn engine oil filter is a filter whose function is to filter and remove contaminants that may be present in the engine oil, transmission oil, lubricating oil, or hydraulic oil in order for proper functioning of the engine.
Therefore, the object and description that matches is Object 2 and T.
Object 1 has no matching description.
Learn more about filters and their uses at: https://brainly.com/question/10719424
(Please help 15 points the assignment is late) (multiple questions) (everything is virtual task )
Part 1 What do you think will happen when an effervescent tablet is placed in the water under each inverted glass?
Part 2 Note the changes in the water level and the air space in both glasses, and write down your observations.
Part 3 Did your prediction match your observation?
Part 4. When a substance easily dissolves in a liquid, it has high solubility in that liquid. When a substance does not easily dissolve in a liquid, it has low solubility in that liquid. Solubility of a gas in water describes how well the liquid can “hang on” to gas, instead of releasing it into the air. Based on the results of your experiment, do you think that CO2 has a higher solubility in hot water or cold water? Why?
Part 5 Soda pop is carbonated with CO2. Mark puts one bottle of soda pop in the refrigerator and leaves the other out in the hot sunlight. After one hour, he opens both bottles. Which bottle will likely have more fizzing and bubbles? Why?
Part 6 One result of climate change is that ocean temperatures are increasing. If the temperatures continue to rise, what effect will that have on the oceans’ ability to retain CO2? How might this change affect the atmosphere?


Answers
The dissolution of gases in water is exothermic therefore oceans will dissolve less CO2 as their temperatures rise.
What is effervescence?
The term effervescence refers to the rise of a gas in a system or simply put, it is the evolution of gas. When an effervescent tablet is placed in the water under each inverted glass, a gas will rise in each glass.
The air space in the hot glass is greater than the air space in the cold glass We can see that the gas has a lower solubility in hot water than in cold water. This is because, solubility of gases is exothermic.
The bottle that is left in the hot sun will have more fizzing because the CO2 does not dissolve owing to the higher temperature.
The rise in ocean temperature means that the oceans will dissolve less CO2 in the future and reduce the CO2 holding capacity of the oceans.
Learn more about CO2: https://brainly.com/question/867804?
can you get pur alcohol from the mixture of alcohol and water using simple distillation process ? explain the process.
Answers
Which of the following is not a possible sublevel?
a. 1s
b. 2p
c. 3f
d. 4d
Answers
Reason: For principle quantum level 3, there are only s and p orbitals that can exist, you can refer to the four quantum numbers concept to understand this further.
Considering the activity series given below for metals and nonmetals, which reaction will occur? Al > Mn > Zn > Cr > Fe > Cd > Co > Ni > Sn > Pb > H > Sb > Bi > Cu > Ag > Pd >Hg > Pt F > Cl > Br > I
Answers
Given the activity series of elements, the reaction that will occur is: 2AgNO3 + Ni -----> Ni(NO3)2 + 2Ag
The activity series of elements can also be called the "reactivity series" of elements because it actually arrangement of elements in order of decreasing reactivity. The most reactive elements are higher up in the series while the less reactive elements are found at the bottom of the series.
Given the fact that nickel is far higher in the series than silver, nickel can displace silver from an aqueous solution of its salt. Hence the equation; 2AgNO3 + Ni -----> Ni(NO3)2 + 2Ag is possible.
Learn more: https://brainly.com/question/8053373

Answer:
Option (C) = 2AgNO3 + Ni Right arrow. Ni(NO3)2 + 2Ag
Explanation:
EDG22, pls tell me if im wrong!
In the reaction of an alkyl bromide with sodium iodide in acetone, why would the resulting alkyl iodide be attacked by a bromide ion?
Answers
In the reaction of an alkyl bromide with sodium iodide in acetone, the resulting alkyl iodide may be attacked by a bromide ion due to a possible nucleophilic substitution reaction. During the initial reaction, the sodium iodide reacts with the alkyl bromide to form an alkyl iodide and sodium bromide.
However, if there is excess alkyl bromide present, the resulting alkyl iodide may undergo a second substitution reaction with the excess alkyl bromide acting as the nucleophile. This can occur because the alkyl iodide is still reactive and can be attacked by the bromide ion, which is also present in the reaction mixture. The resulting product would be a mixed alkyl halide containing both iodine and bromine.
Find out more about alkyl bromide
brainly.com/question/31412665
#SPJ11
predict what precipitate will form when a solution of aluminum chloride is mixed with a solution of potassium phosphate. AlPO4NaCl Na3Cl3 Al3PO4
Answers
When a solution of aluminum chloride is mixed with a solution of potassium phosphate, a precipitate of aluminum phosphate (AlPO4) will form. The reaction between aluminum chloride and potassium phosphate can be represented as follows: AlCl3 + K3PO4 → AlPO4 + 3KCl .
This is due to the fact that the aluminum ion (Al3+) and the phosphate ion (PO43-) can react to form a solid precipitate, which is insoluble in water.
Therefore, when a solution of aluminum chloride is mixed with a solution of potassium phosphate, a white precipitate of aluminum phosphate (AlPO4) will form, while potassium chloride (KCl) will remain in solution.
To know more about aluminum phosphate (AlPO4), here
brainly.com/question/15146433
#SPJ4
what includes the carbon dioxide and carbon monoxide in the atmosphere, produced by business processes and systems?
Answers
Carbon emissions includes the carbon dioxide and carbon monoxide in the atmosphere, produced by business processes and systems.
What does carbon emission mean?
Carbon dioxide emissions or CO2 emissions are emissions stemming by the burning of fossil fuels and the manufacture of cement; they contain carbon dioxide produced during consumption of solid, liquid, and gas fuels also as gas flaring.
How does carbon emission work?A drive to figure , a flip of a light-weight switch and a flight out of town all rely on the combustion of fossil fuels like oil, coal and gas. When fossil fuels burn, they emit greenhouse gases like CO2 that contribute to heating . Ninety-eight percent of atmospheric CO2 comes from the combustion of fossil fuels.
Learn more about carbon emission:
brainly.com/question/1301348
#SPJ4
Using a logarithmic concentration diagram, determine the pH of a solution containing 10-2 M acetic acid and 2 x 10-2 M sodium acetate.
Answers
The pH of this solution is approximately 4.74, indicating it is slightly acidic. The presence of sodium acetate, a salt of acetic acid, acts as a buffer and helps maintain the pH of the solution.
The pH of a solution containing\(10^-2\) M acetic acid and 2 x\(10^-2\) M sodium acetate can be determined using a logarithmic concentration diagram.
To determine the pH of the solution, we need to consider the dissociation of acetic acid and the hydrolysis of sodium acetate. Acetic acid (CH3COOH) is a weak acid that partially dissociates in water, releasing hydrogen ions (H+) and acetate ions (CH3COO-).
The dissociation of acetic acid can be represented as follows:
CH3COOH ⇌ H+ + CH3COO-
The equilibrium constant for this dissociation is known as the acid dissociation constant (Ka). The pKa value of acetic acid is approximately 4.74. The pKa is the negative logarithm of the Ka value.
In the given solution, we have both acetic acid and sodium acetate. Sodium acetate (CH3COONa) is a salt that dissociates completely in water, releasing sodium ions (Na+) and acetate ions (CH3COO-). The acetate ions from sodium acetate can react with any additional H+ ions present in the solution through hydrolysis, which helps maintain the pH.
Using a logarithmic concentration diagram, we can determine that the pH of the solution containing \(10^-2\) M acetic acid and 2 x \(10^-2\) M sodium acetate is approximately 4.74, which is slightly acidic.
The presence of sodium acetate acts as a buffer, helping to resist changes in pH by absorbing excess H+ ions or releasing additional H+ ions as needed to maintain the pH within a certain range.
Learn more about logarithmic concentration from the given link:
https://brainly.com/question/3072484
#SPJ11
How many meters are present in the 12.45 miles? Please show your work, and report your answer with the correct number of significant figures and appropriate units.
Answers
Explanation:
We know that,
1 mile = 1609.34 m
We need to find how many meters are present in the 12.45 miles. To find it use unitary method as follows :
12.45 mile = 1609.34 × 12.45
12.45 mile=20036.283 meters
or
\(12.45\ mile=2.0036\times 10^4\ m\)
Hence, this is the required solution.
Write the equation of each circle. center at (4, 3), radius 9
Answers
Answer:
Explanation:
c
After glycogen reserves are depleted what are the major gluconeogenic precursors of glucose under the conditions of
A. starvation
B. intense exercise
Answers
How do you write this number using digits?
eleven million seven hundred forty-nine thousand four hundred thirty-two
Answers
An old refrigerator is rated at 500 W how many kilowatt hours of electric energy what does refrigerator use in 30 days assume the refrigerator is running 12 hours per day
Answers
The refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
To calculate the kilowatt-hours (kWh) of electric energy used by the refrigerator in 30 days, we need to multiply the power rating by the total running time.
Given:
Power rating of the refrigerator = 500 W
Running time per day = 12 hours
Number of days = 30
First, we need to convert the power rating from watts to kilowatts:
Power rating = 500 W / 1000 = 0.5 kW
Next, we calculate the total energy used in kilowatt-hours (kWh) over the 30-day period:
Energy used = Power rating × Running time × Number of days
Energy used = 0.5 kW × 12 hours/day × 30 days
Energy used = 180 kWh
Therefore, the refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
For more question on energy
https://brainly.com/question/29339318
#SPJ8
Determine the number of atoms in 0. 50 moles of zinc
Answers
The number of atoms in 0. 50 moles of zinc is 3.01 x 10 ²³
The number of atoms in one particular carbon isotope, carbon-12, serves as the basis for the idea of the "mole." A mole of carbon atoms, or 6.02 10²³ carbon atoms, can be found in 12g of carbon-12.
The remaining atoms on the periodic table are identified by their molar masses, which indicate how many grams of each are required to make a mole, or 6.02 x 10 ²³atoms, of that element.
Zinc will have 6.02 x 10²³ if it has 1 mole of zinc. In chemistry, we love to cancel units, therefore we simply do so to address your issue.
The number of atoms in one particular carbon isotope, carbon-12, serves as the basis for the idea of the "mole." A mole of carbon atoms, or 6.02 10 23 carbon atoms, can be found in 12g of carbon-12.
The remaining atoms on the periodic table are identified by their molar masses, which indicate how many grams of each are required to make a mole, or 6.02 10 23 atoms, of that element.
Zinc will have 6.02 x 10 ²³ if it has 1 mole of zinc. In chemistry, we love to cancel units, therefore we simply do so to address your issue.
number of Zinc atoms= 0.50 moles x 6.02 x 10 ²³ = 3.01 x 10 ²³
Learn more about number of atom at https://brainly.com/question/30282381
#SPJ4
A crab has an exoskeleton. The combusting wax in a candle wick is an exothermic reaction. What do you think the prefix “exo” means and how does this apply to the burning wax?
Answers
barium has a density of and crystallizes with the body-centered cubic unit cell. calculate the radius of a barium atom.
Answers
The radius of a barium atom is 2.68 Å.
Barium is a chemical element that is silvery-white in color and can be found in the periodic table with the symbol Ba.
The density of barium is 3.51 g/cm3, and it has a body-centered cubic unit cell that crystallizes.
Determine the length of the edge of the unit cell. Since barium has a body-centered cubic unit cell, the length of the edge of the unit cell:
Edge length = 4r /√3
Where r is the radius of the atom.
The volume of the unit cell using the formula:
Volume of unit cell = Edge length³
The number of atoms present in the unit cell. Since barium has a body-centered cubic unit cell, the number of atoms present in the unit cell :
Number of atoms in the unit cell = 2
The mass of a single atom of barium using the formula:
Mass of a single atom = Density of barium / Avogadro’s number
Where Avogadro’s number is 6.02 × 10²³ atoms/mol.
The radius of a barium atom using the formula:
Radius of barium atom = (3 × volume of unit cell / 4 × π × number of atoms in the unit cell)¹/³
The radius of the barium atom :
Edge length = 2r√3
r = (Edge length) / 2√3
Volume of the unit cell = (Edge length)³
Number of atoms in the unit cell = 2
Mass of a single atom = Density of barium / Avogadro’s number
Radius of barium atom = (3 × volume of unit cell / 4 × π × number of atoms in the unit cell)¹/³
Therefore, the radius of a barium atom is 2.68 Å.
to know more about barium refer here:
https://brainly.com/question/30888146#
#SPJ11
What change takes place in a substance as the molecular motion of that substance increases? Responses A. The substance changes from a liquid to a gas. A. The substance changes from a liquid to a gas. B. The substance changes from a gas to a liquid. B. The substance changes from a gas to a liquid. C. The substance changes from a liquid to a solid. C. The substance changes from a liquid to a solid. D. The substance changes from a gas to a solid.
Answers
The distance between atoms widens as their vibrations get more rapid. The substance's state of matter is determined by the movement and spacing of its particles. The thing grows or enhanced molecular mobility.
What causes molecules inside a substance to move differently?Because kinetic energy of a liquid's molecules rises as the temperature does. As a result, the molecules have more flexibility to travel across larger volumes as the forces that attraction between them are eventually overcome.
What is required to promote molecular motion?According to the gas kinetic theory, as a gas's temperature rises, the typical kinetic energy of its molecules rises, leading to more motion. This real gases equation PV=NkT predicts that the increased velocity will increase the gas's outer pressure.
To know more about atoms visit:
https://brainly.com/question/30898688
#SPJ1
What is polarity as used in magnets?
Answers
In a permanent magnet, a magnetic field is produced by the composite motions of electrons in geometrically aligned atoms.
A magnetic field is characterized by poles called north and south. Magnetic polarity refers to the orientation of these poles in space.
Answer:
The polarity of a magnet usually means the direction of a vector which points from the south pole of the magnet toward the north pole. ... In this case the polarity is expressed in a way that indicates a circular direction, such as clockwise, or as a perpendicular vector representing the curl of the internal field.
Explanation:
1.) Describe the two diagrams of a bottled carbonated beverage shown below as greater
pressure or lower pressure, and then as greater solubility or lower solubility. How do these two
examples illustrate the relationship between the solubility of a gas and its vapor pressure? Explain
2.) On the diagrams below, assume that each beaker contains an equal number of moles
of solute. Label each solution as concentrated or dilute. Then indicate the approximate
relative volumes of each solution by describing the surface level on each beaker.
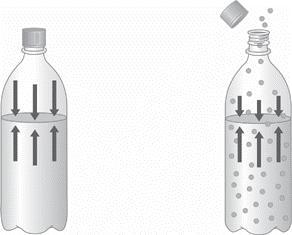

Answers
Answer:
SEE EXPLANATION
Explanation:
If i refer to the bottle at the left hand side as A and the bottle at the right hand side as B. I can say that the bottle A contains solutes which are more soluble than the solutes in bottle B. consequently, A has a lower vapour pressure than B.
We can also see that the pressure in A is much higher than in B(as indicated by the length of the arrows). The higher the pressure, the greater the solubility of the gas. Henry's Law states that: The solubility of a gas in a liquid is directly proportional to the pressure of that gas above the surface of the solution.
Again let me call the beaker on the left A and the beaker on the right B. We can see that the beaker on the left contains 10 ml of solution while the beaker on the right contains 50 ml of solution. It follows that beaker A contains a more concentrated solution since that much amount of solute is contained in a smaller volume than in beaker B as shown in the image.
A sample of nitrogen gas occupies 9.20 L at 21degrees and 0.999m the pressure is decreased to 0.802 am at constant temperature, what is the
newly occupied volume?
Answers
Answer: The newly occupied volume is 11.46 L
Explanation:
Boyle's Law: This law states that pressure is inversely proportional to the volume of the gas at constant temperature and number of moles.
\(P\propto \frac{1}{V}\) (At constant temperature and number of moles)
\(P_1V_1=P_2V_2\)
where,
\(P_1\) = initial pressure of gas = 0.999 atm
\(P_2\) = final pressure of gas = 0.802 atm
\(V_1\) = initial volume of gas = \(9.20 L\)
\(V_2\) = final volume of gas = ?
\(0.999\times 9.20=0.802\times V_2\)
\(V_2=11.46L\)
Therefore, the newly occupied volume is 11.46 L
Calculate the molar solubility of BaCrO4 (Ksp = 2.1 x 10^-10) in each of the following. (a) pure water (b) 1.6 x 10^-3 M Na_2CrO_4
Answers
The molar solubility of BaCrO4 in 1.6 x 10^-3 M Na2CrO4 is 5.25 x 10^-10 M.
The solubility of BaCrO4 can be calculated using the solubility product constant (Ksp) and the stoichiometry of the balanced chemical equation for the dissolution of BaCrO4 in water.
The balanced chemical equation for the dissolution of BaCrO4 in water is:
BaCrO4(s) ↔ Ba2+(aq) + CrO42-(aq)
The solubility product constant (Ksp) expression for this reaction is:
Ksp = [Ba2+][CrO42-]
(a) To calculate the molar solubility of BaCrO4 in pure water:
Ksp = [Ba2+][CrO42-] = (x)(x) = x^2
where x is the molar solubility of BaCrO4 in pure water.
Rearranging the equation and solving for x, we get:
x = sqrt(Ksp) = sqrt(2.1 x 10^-10) = 1.45 x 10^-5 M
Therefore, the molar solubility of BaCrO4 in pure water is 1.45 x 10^-5 M.
(b) To calculate the molar solubility of BaCrO4 in 1.6 x 10^-3 M Na2CrO4:
In this case, the dissolution of BaCrO4 is affected by the common ion effect due to the presence of CrO42- ions from Na2CrO4. The balanced chemical equation for the reaction is:
BaCrO4(s) + 2Na+(aq) ↔ Ba2+(aq) + CrO42-(aq) + 2Na+(aq)
The initial concentration of CrO42- ions is 1.6 x 10^-3 M, and the concentration of BaCrO4 is x. Therefore, the equilibrium concentration of CrO42- ions is (1.6 x 10^-3 + x) M, and the equilibrium concentrations of Ba2+ and CrO42- ions are both x M.
The Ksp expression for the reaction is:
Ksp = [Ba2+][CrO42-] = (x)(1.6 x 10^-3 + x)
Substituting the value of Ksp and solving for x using the quadratic formula, we get:
x = 5.25 x 10^-10 M
Therefore, the molar solubility of BaCrO4 in 1.6 x 10^-3 M Na2CrO4 is 5.25 x 10^-10 M.
Visit here to learn more about stoichiometry brainly.com/question/28780091
#SPJ11
You can bake food in either a metal pan or an oven safe glass. Which would require more energy to heat up? Which would cool down the fastest? Explain your reasoning.
Answers
The kind of material used to bake food will influence how much energy is required to heat it up and how quickly it cools. Due to the fact that metal conducts heat more effectively than glass, heating up metal pans often takes longer than heating up glass ovenware.
What is the behavior of metal with the heat ?This implies that the metal absorbs heat from the oven more rapidly and effectively, needing more energy to attain the appropriate temperature. However, oven-safe glass will cool down more quickly than metal.
This is due to the fact that glass is a poor heat conductor, which means it takes more time for the heat to be absorbed by the glass. The glass will shatter after the dish is cooked and the heat is turned off.As the heat takes longer to dissipate, the metal cools down more quickly than the other materials.
In conclusion, glass cools more quickly than metal and requires less energy to heat up than oven-safe metal. This is because metal absorbs heat from the oven more rapidly and effectively than glass since metal is a stronger heat conductor than glass.
However, because glass is a poor conductor of heat, it takes longer for the food to cook and the heat to be absorbed. The glass cools down more quickly than the metal when the heat is gone.
Learn more about conductor at:
https://brainly.com/question/14405035
#SPJ3
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
Where can one find most of the world's drinking water? Question 4 options: freshwater collection areas. rain clouds , manmade reservoirs , oceans
Answers
Fresh water collection areas is the area where you can find most of the
world's drinking water.
What is Freshwater?
This is the type of water which contains low amount of dissolved salts and
solid particles which makes it very suitable for drinking.
Examples of Freshwater collection areas include the following:
RiversLakes StreamsGroundwaterRead more about Freshwater here https://brainly.com/question/20458567
how to make a pb&j in 30 steps
Answers
this was great and fun to do

HELP PLZZ i think the answer is either C or D.
Scientists hypothesize Earth’s early atmosphere was a result of outgassing from the interior of the planet. Gravity trapped the gasses (including NH3 and CO2) close to Earth’s surface. Over time, bacteria with the ability to perform photosynthesis changed the composition of the atmosphere. As plants evolved, levels of oxygen continued to increase as the levels of carbon dioxide decreased. The hydrosphere – primarily the ocean – played a major role absorbing carbon dioxide from the atmosphere. As plants grew on Earth’s surface, they weathered the geosphere producing soil. Microorganisms in the soil allowed for the evolution of land plants which also extended the boundaries for early animal populations.
As outgassing and volcanic eruptions created Earth’s early atmosphere, what two spheres directly affected one another?
A) Biosphere and geosphere
B) Hydrosphere and biosphere
C) Geosphere and atmosphere
D) Atmosphere and hydrosphere
Answers
Answer:
hi I believe the answer is (C)
Explanation:
I'm not 100%, sure if (C) is the right answer sorry I just have a feeling that's it (C) and also after I did a little tad bit of research but I'm sorry if it isn't right. ( well I'm going to go to sleep.,. I hope you get a good grade in your assignment:>)
If aluminum nitrate reacts with calcium phosphite, what is the balanced coefficient of aluminum nitrate?
Answers
Answer:
The coefficient of aluminum nitrate is 2.
Explanation:
The reaction between aluminum nitrate and calcium phosphite is:
Al(NO₃)₃ + CaHPO₃ → Al₂(HPO₃)₃ + Ca(NO₃)₂
Now, we need to balance the equation. Let's begin with phosphite anion. On the reactant side we have 1 molecule of the anion, and on the products side we have 3 of it, so we need to add a coefficient of 3 before CaHPO₃:
Al(NO₃)₃ + 3CaHPO₃ → Al₂(HPO₃)₃ + Ca(NO₃)₂
The number of Ca atoms on the reactants side is now 3 and on the products side is 1, so we need to add a coefficient of 3 as follows:
Al(NO₃)₃ + 3CaHPO₃ → Al₂(HPO₃)₃ + 3Ca(NO₃)₂
The reactants side has 1 atom of Al, and the products side has 2. Let's add a coefficient of 2 on the reactant side:
2Al(NO₃)₃ + 3CaHPO₃ → Al₂(HPO₃)₃ + 3Ca(NO₃)₂
Finally, the nitrate anion has 6 molecules on the reactant side, which is equal to the number of molecules of NO₃⁻ on the product side.
Hence, in the balanced equation, we have a coefficient of 2 in aluminum nitrate.
I hope it helps you!