Answers
Answer:
I believe H2.
Explanation:
It's H2 because there are two electrons on there being share by 2 atoms.
Since Hydrogen can only get a maximum of 2 electrons and no other atoms are present, that is why it's H2.
Related Questions
Balancing chemical reactions. What is it and examples
Answers
Answer: A balanced chemical reaction is A balanced chemical equation where the number of atoms of each type in the response is the same on both reactants and product sides.
Explanation: Burning, and cooking are 2 examples.
Repeated trials and replication are the same thing.
Group of answer choices
True
False
Answers
Answer:
false
Explanation:
What is the normality of a solution of sulfuric acid (H2SO4) that containing 86 g
per liter of solution?
Answers
Normality of a solution of sulfuric acid (H2SO4) that containing 86 g
per liter of solution is 1N
What is normality?The gram equivalent weight of a solute per liter of solution is what is known as a solution's normalcy. The comparable concentration is another name for it. The units of concentration are denoted by the symbols N, eq/L, or meq/L (= 0.001 N). A hydrochloric acid solution's concentration, for instance, could be stated as 0.1 N HCl. The reactive capacity of a specific chemical species is measured by gram equivalent weight or equivalent (ion, molecule, etc.). Utilizing the chemical species' molecular weight and valence, the equivalent value is calculated. The only concentration unit that is reaction-dependent is normality.
g equivalent mass of H2SO4 = 86g
Volume of solution in liter = 1 l
number of g equivalent of H2SO4 = 86/86 = 1
Normality = number of g equivalent/ volume of solution in liter
N = 1 / 1
Normality = 1 N
To know more about normality, visit:
https://brainly.com/question/14945213
#SPJ13
Which of these compounds has an empirical formula of CH3?
1. C2H5
2.C13H26
3.C26H78
4.C36H110
Answers
Answer:
B I had the same queastion as this one
Please Help me solve for B
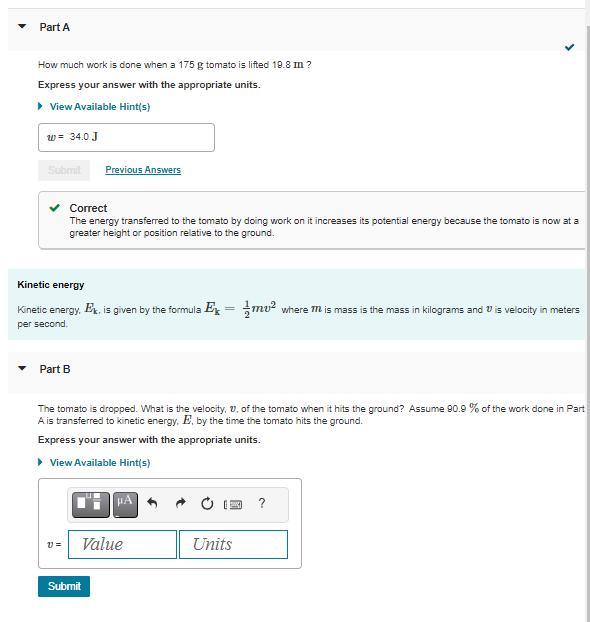
Answers
The velocity of the tomato when it hits the ground is approximately 13.49 meters per second.
The potential energy of the tomato is at the height of 10 meters. When the tomato hits the ground, most of the potential energy is E1 = 0.909*mgh.
By the conservation of energy principle, the kinetic energy \(E_1\) is equal to the kinetic energy \(E_2\) of the tomato just before it hits the ground.
The kinetic energy \(E_2\) is given by\(1/2mv^2\), where v is the velocity of the tomato just before it hits the ground. Equating \(E_1\) and \(E_2\) solving for v, we get:
\(v = \sqrt{(20.909gh)\)
Substituting the values of \(g = 9.81 m/s^2\)and h = 10 m, we get:
v = \(\sqrt{(20.9099.81*10)}\) = 13.49 m/s
To know more about potential energy, here
brainly.com/question/24284560
#SPJ1
--The complete Question is, Suppose a tomato is dropped from a height of 10 meters. If 90.9% of the work done on the tomato is converted to kinetic energy by the time it hits the ground, what is the velocity (in meters per second) of the tomato when it hits the ground? --
If 22.00 mL of 2.00 M potassium iodide is needed to reach the equivalence point with 18.00 mL of lead (II) nitrate, determine the molarity of the lead (II) nitrate solution. Note: First write the balance equation between potassium iodide and lead (II) nitrate.
Answers
The molarity of the lead(II) nitrate solution if 22.00 mL of 2.00 M potassium iodide is needed to reach the equivalence point with 18.00 mL of lead (II) nitrate is 1.2M.
How to calculate molarity?The molarity of a solution can be calculated using the following formula:
CaVa/CbVb = na/nb
Where;
Ca = concentration of acidCb = concentration of baseVa = volume of acidVb = volume of basena = number of moles of acidnb = number of moles of baseThe balanced equation of the reaction is as follows:
Pb(NO3)2 (aq) + 2KI (aq) → 2KNO3 (aq) + PbI2 (s)
22 × 2/18 × Cb = 2/1
44/18Cb = 2
Cb = 44 ÷ 36
Cb = 1.2M
Therefore, the molarity of the lead(II) nitrate solution if 22.00 mL of 2.00 M potassium iodide is needed to reach the equivalence point with 18.00 mL of lead (II) nitrate is 1.2M.
Learn more about molarity at: https://brainly.com/question/356585
What changes sodium pellets to liquid
Answers
Answer:
when placed in water, a sodium pellet catches on fire as hydrogen gas is liberated and sodium hydroxide forms. chemical change = fire is a sign of chemical reaction.
Explanation:
When placed in water the sodium pellets catch the fire and liberate the hydrogen gas. On mixing with water solid sodium forms a colorless basic solution.
What are the properties of sodium?Sodium is a soft metal. It is a very reactive element with a low melting point. Sodium reacts very quickly with water, snow, and ice to produce sodium hydroxide and hydrogen. It is an alkali metal and the sixth most abundant metal on earth. It has a silvery white color.
It has a strong metallic luster. On reacting with oxygen it produces sodium oxide which on reacting with the water produces sodium hydroxide.
It is used to improve the structure of certain alloys and soaps. It is also used in the purification of metals. Sodium is also present in sodium chloride, an important compound found in the environment.
To learn more about sodium, refer to the link:
https://brainly.com/question/29327783
#SPJ2
In using the Haber process in the formation of ammonia, what mass of hydrogen is needed to produce 51.0 grams of ammonia? 3 H₂(g) + N2 (g) → 2 NH3(g).
Answers
The mass of hydrogen needed to produce 51.0 grams of ammonia is ≈ 9.07 grams.
To determine the mass of hydrogen required to produce 51.0 grams of ammonia (NH3) using the Haber process, we need to calculate the stoichiometric ratio between hydrogen and ammonia.
From the balanced chemical equation:
3 H₂(g) + N₂(g) → 2 NH₃(g)
We can see that for every 3 moles of hydrogen (H₂), we obtain 2 moles of ammonia (NH₃).
First, we need to convert the given mass of ammonia (51.0 grams) to moles. The molar mass of NH₃ is 17.03 g/mol.
Number of moles of NH₃ = Mass / Molar mass
= 51.0 g / 17.03 g/mol
≈ 2.995 moles
Next, using the stoichiometric ratio, we can calculate the moles of hydrogen required.
Moles of H₂ = (Moles of NH₃ × Coefficient of H₂) / Coefficient of NH₃
= (2.995 moles × 3) / 2
≈ 4.493 moles
Finally, we can convert the moles of hydrogen to mass using the molar mass of hydrogen (2.02 g/mol).
Mass of H₂ = Moles × Molar mass
= 4.493 moles × 2.02 g/mol
≈ 9.07 grams
Therefore, approximately 9.07 grams of hydrogen is needed to produce 51.0 grams of ammonia in the Haber process.
Know more about the mass of hydrogen here:
https://brainly.com/question/14083730
#SPJ8
Why is it important to have only one independent variable and to control the
other variables when performing science experiments
Answers
Scientists cannot pinpoint the changes or variations in the results to a single source when more than one variable is altered during an experiment. The outcomes can be attributed to the independent variable directly by examining and modifying each variable separately.
You can evaluate the outcomes of your experiment to determine how much one adjustment impacted the outcome by testing just one variable at a time. You won't be able to identify the variable that caused the outcome if you're testing two variables simultaneously. A controlled variable is one that is kept constant with the goal of not affecting how an experiment turns out. Controlling any factors that can affect an experiment's outcomes allows us to be certain that the altered variable is what caused our results.
Learn more about variables here-
https://brainly.com/question/17344045
#SPJ9
Hydrogen gas is collected over water at 23°C, 767 torr. At this temperature the vapor pressure of water is 21.0 torr. What is the partial pressure of hydrogen in the collected gas?
Answers
The partial pressure of hydrogen gas in the collected gas is 746 torr.
To determine the partial pressure of hydrogen gas in the collected gas, we need to consider the difference between the total pressure of the collected gas and the vapor pressure of water at the given temperature. The partial pressure of hydrogen gas is the pressure exerted by hydrogen alone.
Given information:
Total pressure of the collected gas (Ptotal) = 767 torr
Vapor pressure of water (Pwater) = 21.0 torr
The partial pressure of hydrogen gas (Phydrogen) can be calculated using Dalton's law of partial pressures, which states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each individual gas.
Phydrogen = Ptotal - Pwater
Plugging in the given values:
Phydrogen = 767 torr - 21.0 torr
Phydrogen = 746 torr
Therefore, the partial pressure of hydrogen gas in the collected gas is 746 torr.
It's important to note that in this calculation, we assume that the water vapor does not react with or dissolve in the hydrogen gas and that the gases behave ideally. Additionally, it's assumed that the collected gas is dry, meaning all the water vapor has been removed or does not significantly contribute to the total pressure.
Fo rmore such questions on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
what type of sugar has the largest surface area
a) powered/icing
b) raw
c) cubed
d) granulated
Answers
Answer:
d.) granulated
Because the granulated sugar has a greater surface area.
What is the percent m/m concentration of an aqueous solution of sodium nitrate in which there are 24.34 grams of solute in 138.87 grams of solvent
Answers
Answer:
14.9%m/m
Explanation:
The percent m/m concentration of a solution (%m/m) indicates the grams of solute there are in 100 g of solution. It can be calculated as follows:
\(%m/m= \frac{mass of solute (g)}{mass of solution(g)} x 100\)%m/m= mass of solute(g)/mass of solution(g) x 100
As the solution is composed by solute + solvent, the mathematical expression can be transformed in:
\(%m/m= \frac{mass of solute (g)}{mass of solution(g)} x 100\)%m/m= mass of solute(g)/(mass of solute + mass of solvent (g) )x 100
= 24.34 g\(%m/m= \frac{mass of solute (g)}{mass of solution(g)} x 100\)/(24.34 g + 138.87 g) x 100
= 24.34 g/(163.21 g) x 100
= 14.9%
\(%m/m= \frac{mass of solute (g)}{mass of solution} x 100 = \frac{mass of solute (g)}{mass of solute (g) + mass of solvent (g)}\)
A student, Ken, is given a mixture containing two carbonate compounds. The mixture includes MgCO3 and (NH4)₂CO3. The
mixture is 68.55% CO3 is by mass. What is the mass percent of MgCO3 in the mixture?
Answers
Answer:
Let's assume that the mass of the mixture is 100 g. Then, the mass of CO3 is 68.55 g.
Let's represent the mass of MgCO3 as x g. Then, the mass of (NH4)2CO3 will be (100 - x) g.
From the molecular formula of MgCO3, we know that the mass percent of CO3 in MgCO3 is 60/84 = 0.7143 or 71.43%.
Similarly, from the molecular formula of (NH4)2CO3, we know that the mass percent of CO3 in (NH4)2CO3 is 60/96 = 0.625 or 62.5%.
Therefore, the total mass percent of CO3 in the mixture is given by the weighted average of the mass percent of CO3 in MgCO3 and (NH4)2CO3, where the weights are the masses of the respective compounds in the mixture.
This can be expressed as:
68.55 = (0.7143)x + (0.625)(100 - x)
Simplifying and solving for x, we get:
x = 47.1 g
Therefore, the mass of MgCO3 in the mixture is 47.1 g, and the mass percent of MgCO3 in the mixture is:
(47.1/100) x 100% = 47.1%
is magnesium oxide a metal
Answers
Answer:
Magnesia, or magnesium oxide, is a mineral composed of alkaline earth metals. The majority of today's magnesium oxide comes from calcining natural minerals.
Explanation:
I hope this helps you
:)
60 points!! Look at picture please don’t troll
Answers
Please if you know the answer put it thanks

Answers
The diagram shows a picture of a compound.
What is a compound?A compound is a substance that is made up of two or more different elements that are chemically bonded together in a specific ratio.
This means that the elements are combined in a way that creates a new substance with different physical and chemical properties than the individual elements.
Compounds can be formed through a variety of chemical reactions, such as combining elements through a chemical bond or through a reaction between an acid and a base.
So for the given diagram, we can see that it represents two or more elements chemically combined.
Learn more about a compound here: https://brainly.com/question/29108029
#SPJ1
A certain element has 12 neutrons and 12 protons. Assume that this atom has an
overall neutral charge.
What is the identity of this element?
Answers
The chemical formula of ethanol is C₂H5OH. How many atoms are in 1.73 mol of ethanol?
Answers
Ethanol has the chemical formula C2H5OH. There will be twice as many carbon atoms as molecules, or two at a time 7.65 times Ten to something like a total of around 23.
What is the purpose of ethanol?When diluted, alcoholic beverages contain ethanol. It is used as a topical therapy to combat staph infections as well as in cosmetics, fragrances, and medicinal compounds. Bioethanol, ethanol, and denatured alcohol are examples of monohydric solvents, each of which has just one hydroxyl group.
Exactly how is ethanol made?Corn is the most widely used ingredient in ethanol produced domestically. It can also be produced utilizing cellulosic feedstocks such crop waste and wood, however this is less common. The majority of ethyl acetate plants in the US have been situated in the Midwest due to their proximity.
To know more about ethanol visit:
https://brainly.com/question/15176311
#SPJ1
Express the number 4.80x10-1 in standard form
Answers
Part G
The compound Iron oxide can exist with either iron(II) ions or Iron(III) ions. Conduct Internet research to learn about the
differences between iron(II) oxide and iron(III) oxide. Give the chemical formula for each compound. Describe their
appearance and uses. Based on your findings, are these two forms of the same compound, or are they two completely
different compounds?
BIU xX² X₂
parameters
chemical formula
appearance
uses
10pt
V
iron(II)oxide
AvZv = = = = = = V V
Iron(III) oxide
Answers
Answer:
Therefore the theoretical density of iron is 7.877 g/cm³ .
Therefore the number of vacancy per cm³ is
Explanation:
The bright-line spectra of four elements, G,J, L, and M, and a mixture of at
least two of these elements are given below.
Which elements are present in the mixture?
M
Mixture
750
750
G and J
G and L
M, J, and G
M, J, and L
700
700
650
650
Bright-Line Spectra
600
600
550 500
550
Wavelength (nm)
500
450
450
400
400
.
Answers
Based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
From the given bright-line spectra and the spectrum of the mixture, we can determine the elements present in the mixture by comparing the specific wavelengths observed. Examining the bright-line spectra, we can identify that G has a distinct wavelength at 650 nm, J at 600 nm, L at 550 nm, and M at 500 nm.
Looking at the spectrum of the mixture, we can observe two prominent wavelengths, 650 nm and 600 nm. These correspond to the wavelengths of G and J, respectively. Since the spectrum of the mixture does not exhibit the wavelengths specific to L (550 nm) or M (500 nm), we can conclude that only G and J are present in the mixture.
Therefore, based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
This analysis relies on the principle that each element has characteristic wavelengths at which they emit light. By comparing the observed wavelengths in the mixture's spectrum with those of the individual elements, we can determine the elements present in the mixture.
Know more about wavelengths here:
https://brainly.com/question/10750459
#SPJ8
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
Describe the composition of humus and why it is an effective organic fertilizer.
Answers
Answer:
In classical soil science, humus is the fraction of soil organic matter that is amorphous and without the "cellular cake structure characteristic of plants
Explanation:
anyways were r we going 2 talk?
and wut did i miss?
A certain mass of hydrogen gas collected over water at 10°C and 750mmHg pressure has a volume of 57cm3. Calculate the volume when it is dry at S.T.P ( S.V.P of water at 10°C = 9.2 mmHg .
Answers
The volume of gas when it is dry at S.T.P : 53.6 cm³
Further explanationGiven
P tot=750 mmHg
V₁=57 cm³
T₁=10 °C = 283 K
P₂ = 760 mmHg(STP)
T₂ = 273 K(STP)
Required
P dry gas at STP
Solution
Dalton's Law
P tot = P H₂O + P gas
P gas = 750 mmHg - 9.2 mmHg
P gas = 740.8 mmHg = P₁
Combined gas Law
\(\tt \dfrac{P_1.V_1}{T_1}=\dfrac{P_2.V_2}{T_2}\\\\V_2=\dfrac{P_1.V_1.T_2}{T_1.P_2}\\\\V_2=\dfrac{740.8\times 57\times 273}{283\times 760}=53.6~cm^3\)
Perform the following operationand express the answer inscientific notation.7.00x105 – 5.00x104-[ ? ]x10[?]Coefficient (green)Exponent (yellow)Enter
![Perform the following operationand express the answer inscientific notation.7.00x105 5.00x104-[ ? ]x10[?]Coefficient](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/4Vkq1ChyWUYhUSIQBQ0MduJeozc8y21b.jpeg)
Answers
The answer is 6.5x10^5
Coefficient = 6.5
Exponent = 5
Which of these pairs of atoms are isotopes?
1. Atom A: 13 protons, 12 neutrons and 12 electrons. Atom B: 12 protons, 12 neutrons and 12 electrons
2. Atom A: 12 protons, 12 neutrons and 12 electrons. Atom B: 12 protons, 12 neutrons and 13 electrons
3. Atom A: 12 protons, 12 neutrons and 12 electrons. Atom B: 12 protons, 13 neutrons and 12 electrons
Answers
Answer:
number 3
Explanation:
In isotopes the number of protons and electrons is always the same since they are isotopes of the same element. If the number of protons in atom "a" is different than in atom "b" then these are 2 different elements.
Isotopes have the same number of electrons and protons but a different number if neutrons.
6.
Which of these does not support the phenomenom
of kinetic theory.
A Brownian motion
B Diffusion
C Osmosis
D Linear expansivity
Kinetic Theory of Matter Gas Laws
Answers
Help for both questions please and thanks

Answers
Answer:
hey can you re post this and zoom in i can see what it say its not allowing me to zoom thx
Explanation:
If the volume of a substance is 1 cubic meter, then what is its
volume in centiliters?
Answers
Answer:
0.1CL
Explanation:
The problem entails we convert from cm³ to centiliters;
To solve this problem:
Convert from cm³ to Liters and then Centiliters.
1000cm³ = 1L
So 1cm³ will give 0.001L
Also;
100CL will give 1 liter;
1Cl = 1 x 10⁻²L
So:
0.01L will give 0.001 x 100 = 0.1CL
From the scenarios described below, which would most likely lead to the formation of a hurricane?
A. a high pressure zone meeting a low pressure zone over warm land
B. thunderstorms meeting over cool ocean water
C. thunderstorms meeting over warm ocean water
D. a warm front and a cool front meet over warm water
Answers
Answer:
D.
Explanation:
Hurrocanes are caused from un wanted mixed weather
