what is the equation for reaction
Answers
Answer:
A chemical reaction equation gives the reactants and products, and a balanced chemical reaction equation shows the mole relationships of reactants and products. Often, the amount of energy involved in the reaction is given. Dealing with the quantitative aspect of chemical reactions is called reaction stoichiometry.
Related Questions
5) Calculate the volume, in liters, of 3.24 x 1022 molecules
CI2
Answers
Answer: 1.12 Liters
Explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number \(6.023\times 10^{23}\) of particles.
To calculate the moles, we use the equation:
\(\text{Number of moles}=\frac{\text{Given molecules}}{\text {avogadro's number}}=\frac{3.24\times 10^{22}}{6.023\times 10^{23}}=0.05moles\)
1 mol of \(Cl_2\) occupy = 22.4 L
Thus 0.05 mol of \(Cl_2\) will occupy = \(\frac{22.4}{1}\times 0.05=1.12 L\)
Which statement best describes the rock cycle?
Processes that change rocks from one type to another
A classification system for rocks
A way to figure out the age of rocks
A process in which the air changes the types of rocks
Answers
Answer: Processes that change rocks from one type to another.
Explanation: During the rock cycle, different rock types change from one to another. For example, sedimentary rock changes to metamorphic rock through heat and pressure.
What is the molar mass of an unknown if a 0.45 M solution is created by dissolving 12 grams in 425 mL of water?
Answers
To calculate the molar mass of the unknown substance, we need to use the formula:
Molar mass = (mass of solute) / (number of moles of solute)
First, let's calculate the number of moles of solute in the solution:
Number of moles = (concentration) x (volume in liters)
We know that the concentration of the solution is 0.45 M, and the volume of the solution is 425 mL, which is equivalent to 0.425 L. Substituting these values into the formula, we get:
Number of moles = 0.45 M x 0.425 L
Number of moles = 0.19125 moles
Next, we can calculate the mass of the solute (the unknown substance) by using the formula:
mass = number of moles x molar mass
Rearranging the formula, we get:
molar mass = mass / number of moles
We know that the mass of the solute is 12 grams, and we have already calculated the number of moles as 0.19125 moles. Substituting these values into the formula, we get:
molar mass = 12 g / 0.19125 moles
molar mass = 62.8 g/mol
Therefore, the molar mass of the unknown substance is 62.8 g/mol.
Calculate the power developed in R1.
P1 =_____watts
48
190
240
1300
Answers
the answer will be 190
Explanation:
because R1= 0.5*0.5*8
=190
Write the balanced net ionic equation for the reactions that occur when the given aqueous solutions are mixed. Include the physical states. A. Silver nitrate, agno3 , and magnesium bromide, mgbr2.
Answers
A silver precipitate is created when silver nitrate, AgNO3, and magnesium bromide, MgBr2, are combined in aqueous solutions.Create the net ionic equation as well as the balanced formula equation for this reaction.
When AgNO3 and MgCl2 are combined, does a solid result?The end products of the reaction between aqueous systems of magnesium chloride (MgCl2) and silver nitrate (AgNO3) are solid silver chloride and aqueous magnesium nitrate.
Which one of the following results in an AgCl and AgNO3 precipitate?The right response is (CH3)3C-Cl.The most reliable 3° carbocation is formed by tert-butyl chloride, or (CH3)3C - Cl.As a result, it will immediately produce the white precipitate of AgCl in AgNO3 solution.
To know more about ionic equation visit:
https://brainly.com/question/15467502
#SPJ4
A volume of gas at 1.01 atm was measured at 236 mL . What will be the volume if the pressure is
adjusted to 1.90 atm?
Answers
Answer:
1.01x236/1.90
=125.4mL
What is an action citizens can take to prevent energy waste?
Answers
Answer:
Turning the lights off when they're not needed.
Which of the following radiations is attracted to the positive electrode?
a.Alpha and beta particle
b.Alpha particle and neutron
c.Photon
d.Beta particle
Answers
The balanced chemical equation for an acid-base reaction is
2HCI+ Ca(OH)2 +CaCl₂ + 2H₂O
For this reaction, how many water molecules form when x molecules of CaCl₂ form?
2
twice as many, 2x
half as many.
an equal number, x

Answers
The balanced chemical equation for the acid-base reaction is:
2HCl + Ca(OH)2 → CaCl2 + 2H2O
A balanced chemical equation is a representation of a chemical reaction that shows the relative number of reactant and product molecules involved. It follows the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. In a balanced equation, the number of atoms of each element on both sides of the equation is equal.
A balanced chemical equation includes chemical formulas of reactants on the left side of the arrow and the chemical formulas of products on the right side. Coefficients are used to balance the equation by adjusting the number of molecules or moles of each substance involved. These coefficients indicate the relative stoichiometric ratios between reactants and products.
According to the equation, for every 1 molecule of CaCl2 that forms, 2 water molecules are produced. Therefore, the correct answer is:
twice as many, 2x
For more details regarding balanced chemical equation, visit:
https://brainly.com/question/14072552
#SPJ1
why do we use acid during the detection of halogens
Answers
Answer:
Nitric acid decomposes sodium cyanide and sodium halide. else, they precipitate in test and misguide the result. Therefore, dilute nitric acid is added before testing halogens to expel all the gases if evolved.
SOMEONE HELP ASAPP...

Answers
AnswU GOT THIS!!!!!!!!!!!!!
Explanation:
Answer:
protein??
Explanation:
im not sure, but I could be wrong!!
what type of substances are required for the production of illicit drugs?
Answers
The substances required for the production of illicit drugs vary depending on the type of drug being produced. For example, methamphetamine production typically involves the use of chemicals such as pseudoephedrine, anhydrous ammonia, and lithium, while cocaine production requires coca leaves and other chemicals like hydrochloric acid.
Other illicit drugs like heroin, ecstasy, and LSD also require specific substances for production. It is important to note that the production of illicit drugs is illegal and poses significant health and safety risks.
to know more about substances intake pls visit:
https://brainly.com/question/7482280
#SPJ11
How does electron pair repulsion determine the molecular shape/molecule geometry?.
Answers
The number of valence electron pairs in the outermost shell, as determined by the valence shell electron repulsion theory (VSEPR), determines the molecular shape.
By analyzing the repulsion between bond electron pairs in the outermost electron shell, a process known as the molecular shape can determine the shape of a molecule. Because most physical and chemical properties are influenced by molecular shape, it is crucial to study molecular shape or geometry.
The foundation of VSEPR is minimizing the strength of the electron-pair repulsion surrounding the central atom under consideration. The foundation of the VSEPR theory is the notion that the geometry (shape) of a molecule is primarily determined by the repulsion between the pairs of electrons surrounding a central atom.
Learn to know more about Electron pair repulsion on
https://brainly.com/question/10271048
#SPJ4
pls help me with this question

Answers
Answer:
I think its a and c
Explanation:
im smrt
hope this helps ;)
Carbon-12 contains 6 protons and 6 neutrons and electrons. which subatomic particle, if charged, would change identity of the elements.
A. proton
B. neutron
C. electron
D. all of the elements
Answers
All alkalis are bases but not all bases are alkalis. True or False?
Answers
Answer:
A substance that neutralizes an acid is generally called a base and the basic substances that are soluble in water and alkali and the properties of alkalis are due to these hydroxide ions(OH−). ... - Thus, conclusively it can be said that all alkalis are bases but all bases are not alkali.
Answer:
This is false
Explanation:
it is actually the opposite, all bases don't dissolve in water, but alkali is a base that does dissolve in water. So, alkalis are bases, but not all bases are alkalis
Pinky, message in one of my answers that doesn't have an comment on pls.. i wish i can tlk to u.. but this new system in brainly.. oof
Which organization now serves as the gold standard for comparison of hospital performance on national standards of safety, quality, and efficiency, thereby facilitating transparency and easy access to healthcare information
Answers
The organization that currently serves as the gold standard for comparing hospital performance on national standards of safety, quality, and efficiency, while promoting transparency and easy access to healthcare information, is The Joint Commission (TJC).
The Joint Commission (TJC) is an independent, non-profit organization that accredits and certifies healthcare organizations and programs in the United States. It sets rigorous standards for healthcare quality and safety and conducts regular evaluations to ensure compliance. TJC evaluates hospitals and healthcare facilities based on a wide range of criteria, including patient safety, infection control, medication management, clinical outcomes, and patient experience.
TJC's accreditation and certification programs are highly regarded and widely recognized as indicators of a hospital's commitment to delivering high-quality care. By adhering to TJC's standards, hospitals demonstrate their dedication to meeting national benchmarks for safety, quality, and efficiency. This allows patients, healthcare providers, and other stakeholders to have confidence in a hospital's performance and compare it to established standards.
Learn more about Joint Commission here:
https://brainly.com/question/30326335
#SPJ11
Arsenic, hydraulic fracturing, lead, and PFAS present chemical threats to global drinking water supplies in different ways. For each problem, describe: (a) the origin of exposure, (b) human health consequences, (c) drivers of continued exposure, and (d) examples of modern solutions.
Answers
Arsenic, hydraulic fracturing, lead, and PFAS present chemical threats to global drinking water supplies in different ways.
Let's discuss each of them in detail:
(a) Arsenic - The origin of arsenic exposure is natural deposits or contamination from agricultural or industrial practices. Human health consequences include skin, lung, liver, and bladder cancers. It can also lead to cardiovascular diseases, skin lesions, and neurodevelopmental effects. Drivers of continued exposure include poor regulation and monitoring. Modern solutions include rainwater harvesting and treatment.
(b) Hydraulic fracturing - Hydraulic fracturing involves using a mixture of chemicals, water, and sand to extract natural gas and oil from shale rock formations. The origin of exposure is contaminated surface and groundwater due to the release of chemicals from fracking fluids and other sources. Human health consequences include skin, eye, and respiratory irritation, headaches, dizziness, and reproductive and developmental problems. Drivers of continued exposure include lack of regulation and poor oversight. Modern solutions include alternative energy sources and regulation of the industry.
(c) Lead - Lead contamination in drinking water can occur due to corrosion of plumbing materials. Human health consequences include neurological damage, developmental delays, anemia, and hypertension. Drivers of continued exposure include aging infrastructure and poor maintenance. Modern solutions include replacing lead service lines, testing for lead levels, and implementing corrosion control.
(d) PFAS - PFAS (per- and polyfluoroalkyl substances) are human-made chemicals used in a variety of consumer and industrial products. They can enter the water supply through wastewater discharges, firefighting foams, and other sources. Human health consequences include developmental effects, immune system damage, cancer, and thyroid hormone disruption. Drivers of continued exposure include the continued use of PFAS in consumer and industrial products. Modern solutions include reducing the use of PFAS in products and treatment methods such as granular activated carbon.
To know more about hydraulic fracturing visit:
https://brainly.com/question/31032804
#SPJ11
PLEASE HELPPP!!! Identify the ionic bond

Answers
Answer:
B unless not up to u if u learned about two letters with the - in the middle. dont stress me
What volume of a 4.13 M lithium
nitrate solution would be needed tomake 195 mL of a 1.05 M solution
by dilution?
[?] L LINO3
I’ll give u 100 points if ur answer is correct

Answers
Answer:
0.0496
Explanation:
Well, there’s none!!! It’s just so easy…
Answer: 49.6
Explanation: It actually wants the answer it mL not L.
Which solution will have the greatest boiling point? Remember ionic > covalent AND more ions > less ions
a) 0.5 M C₁₁H₂₂O₁₁
b) 1 M NaCl
c) 0.5 M NaCl
d) 1 M C₁₁H₂₂O₁₁
Answers
b) 1 M NaCl will have the highest boiling point because it has the highest number of solute particles present.
When comparing the boiling points of various solutions, the number of solute particles present in the solution is the most important consideration. Ionic substances tend to have higher boiling points than covalent substances since they contain strong electrostatic forces between the ions.
To determine which of the given solutions has the highest boiling point, we must first consider the number of solute particles present in each of the given solutions.
a) 0.5 M C₁₁H₂₂O₁₁C₁₁H₂₂O₁₁ is a covalent substance that does not ionize in water. Thus, only one molecule of C₁₁H₂₂O₁₁ is present in the solution. As a result, it has the lowest boiling point
.b) 1 M NaClNaCl is an ionic compound, which breaks down into two ions in water: Na+ and Cl-. There are twice as many solute particles in the solution as there are in the 0.5 M C₁₁H₂₂O₁₁ solution. As a result, NaCl has a higher boiling point than 0.5 M C₁₁H₂₂O₁₁.
c) 0.5 M NaCl0.5 M NaCl contains the same number of solute particles as 1 M NaCl. As a result, both solutions have the same boiling point.
d) 1 M C₁₁H₂₂O₁₁C₁₁H₂₂O₁₁ is a covalent substance that does not ionize in water. Thus, only one molecule of C₁₁H₂₂O₁₁ is present in the solution.
for more questions on solute :
https://brainly.com/question/23946616
#SPJ11
The arrangement of particles is most ordered in a sample of
1.
NaCl(aq)
2.
NaCl(l)
3.
NaCl(g)
4.
NaCl(s)
PLEASE HELP
Answers
We have that the arrangement of particles of NaCl is most ordered in a sample of
NaCl(s)
i.e solid NaCl
From the question we are told
The arrangement of particles is most ordered in a sample of
1. NaCl(aq)
2. NaCl(l)
3. NaCl(g)
4. NaCl(s)
NaCl Generally known as sodium chloride or salt exist in four main states as shown
1. NaCl(aq)
2. NaCl(l)
3. NaCl(g)
4. NaCl(s)
Mow in a subject of its arrangement of particles we can see that as its state changes from gaseous through to solid it gains in form and arrangement of particles
Therefore
The arrangement of particles of NaCl is most ordered in a sample of
NaCl(s)
i.e solid NaCl
For more information on this visit
https://brainly.com/question/1641336
How many calories (units of heat energy) per serving is an 18 pound deep fried turkey?
Answers
Answer:
245
Explanation:
BECAUSE THERE ENERGY THEY GIVES THE IMPRESSION OF THE TURKISH
Balance this equation by using the correct coefficient
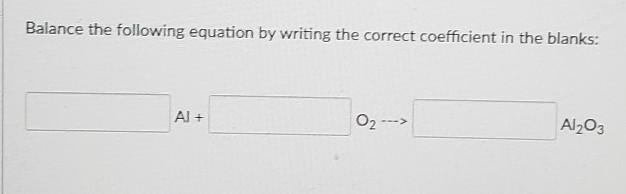
Answers
Answer:
4 Al +3 02-------> 2 AL2O3
Explanation:
Why was periodic table developed
Answers
Answer:
The periodic table was created to organize the elements and provide physical and chemical properties, also to group with elements like it.
Hope this helps
Explain why each of the following statements is false: 1.Reactions with negative reaction free energies occur spontaneously and rapidly. 2.If ΔG^f = 0, the reaction is at equilibrium. 3.Every sample of a pure clement, regardless of its physical state, is assigned a free energy of formation equal to 0. 4.An exothermic reaction producing more moles of gas than arc consumed has a positive standard Gibbs Free energy.
Answers
Reactions with negative reaction free energies occur spontaneously and rapidly is false because it just indicates that the reaction is thermodynamically feasible. If ΔG^f = 0, the reaction is at equilibrium is false because equilibrium is reached when the forward and backward reaction rates are equal.
1. Reactions with negative reaction free energies occur spontaneously and rapidly is false because the Gibbs free energy (ΔG) for spontaneous reactions is always negative, but this doesn't necessarily mean that the reaction will occur rapidly or at a noticeable rate.
2. Statement 2: If ΔG^f = 0, the reaction is at equilibrium.
ΔG^f is the standard free energy of formation. If ΔG^f = 0, it means that the substance is in its most stable state at standard conditions. However, this does not imply that the reaction is at equilibrium.
3. Statement 3: Every sample of a pure element, regardless of its physical state, is assigned a free energy of formation equal to 0
The standard free energy of formation for an element is defined as zero. However, this does not apply to compounds, as their standard free energy of formation is calculated based on the elements from which they are formed.
4. Statement 4: An exothermic reaction producing more moles of gas than are consumed has a positive standard Gibbs Free energy
The standard free energy change of a reaction (ΔG°) is related to the equilibrium constant (K) of the reaction by the equation ΔG° = -RTlnK.
If a reaction is exothermic and produces more moles of gas than are consumed, it will have a negative ΔG°, indicating that the reaction is spontaneous. Therefore, statement 4 is false since a spontaneous exothermic reaction producing more moles of gas than are consumed will have a negative standard Gibbs free energy.
Learn more about element -
brainly.com/question/28376204
#SPJ11
PLEASE HELP I'M SO DOOMED
A balloon that contains 2.8 mol of helium gas has a volume of 31 L. If 3.5 g of helium is added, what will be the new volume of the balloon? Assume the temperature and pressure remain the same. The molar mass of helium is 4.00 g/mol.
a) 186 L
b) 70 L
c) 24 L
d) 41 L
Do not post links
Answers
Answer:
D
Explanation:
31 / 2.8 = 11.0714286 L per mole of helium
3.5 / 4 = 0.875 moles
2.8 + 0.875 = 3.675 moles
11.0714286 x 3.675 = 40.6875 L
Jason took 6 hours to travel 540 km. For the first 140 km, he took 2 hours.
What was his average speed during the first two hours? ___ km/h
How much distance did he cover in the last four hours of his journey? ___ km
What was his average speed for the rest of the journey? ___ km/h
Answers
2. 400 km/h
3. 100 km/h
What is variable????
Answers
Answer:
Basically, a variable is any factor that can be controlled, changed, or measured in an experiment. It's anything that can be changed in the experiment.
Explanation:
Scientific experiments have several types of variables. The independent and dependent variables are the ones usually plotted on a chart or graph, but there are other types of variables you may encounter. The presence/absence of the chemical is the independent variable. The health of the (ex:)rat (whether it lives and can reproduce) is the dependent variable.
Answer:
Variable are all the quantities that could change in an experiment.The relative number of atoms of a compound can be calculated
by dividing the percentage of an element by the:
Answers
Answer:
Obtain the relative numbers of atoms of each element in the compound by dividing the number of moles of each element in the 100 g sample by the number of moles of the element present in the smallest amount.
Answer:
Obtain the relative numbers of atoms of each element in the compound by dividing the number of moles of each element in the 100 g sample by the number of moles of the element present in the smallest amount.