Answers
Among electromagnetic waves, UV rays are most dangerous because exposure to these radiation cause serious problems in living organism. Therefore, 4.28×10⁻¹⁹J is the energy of a photon that emits a light frequency of 6.67×10⁻³⁴ Hz.
What is electromagnetic wave?Electromagnetic wave is a wave which contain two component one is electric component and other is magnetic component. The electric and magnetic component are perpendicular to each other. There are so many wave that comes under electromagnetic wave like infrared wave , radio wave.
There is a relation between energy of wave. frequency of wave, and wavelength of wave
Mathematically,
E=h×ν
where,
E = energy of electromagnetic wave =?
h is planks constant having value 6.67×10⁻³⁴js
ν= frequency of photon=6.42X10¹⁴ Hz
Substituting all the given values in the above equation, we get
E= 6.67×10⁻³⁴×6.42X10¹⁴
E=4.28×10⁻¹⁹J
Therefore, 4.28×10⁻¹⁹J is the energy of a photon that emits a light frequency of 6.67×10⁻³⁴ Hz.
To know more about electromagnetic wave, here:
https://brainly.com/question/12289372
#SPJ1
Related Questions
If 12.7 grams of Carbon combine completely with 17.7 grams of Oxygen to form a compound, what is the percent composition of OXYGEN in the compound?
Answers
The percentage composition or mass percentage is an important method to calculate the concentration of a solution. The percent composition of Oxygen in the compound is 36.79 %.
What is mass percentage?The mass percentage of a particular component in the compound can be defined as the ratio of the mass of that particular component to the total mass of the compound.
% Mass = Mass of the component / Total mass of the compound × 100
Here 'C' and 'O' combines to form CO₂.
Mass of CO₂ = 12.7 + 2 × 17.7 = 48.1 g
% Mass of 'O' = 17.7 / 48.1 × 100 = 36.79 %
Thus the percent composition of oxygen is 36.79 %.
To know more about % Mass, visit;
https://brainly.com/question/8339943
#SPJ1
If the theoretical yield of a reaction is 332.5 g and the percent yield for the reaction is 38 percent, what's the actual yield of product in grams? \
A. 8.74 g
B. 12616 g
C. 116.3 g
D. 126.4 g
Answers
Answer: D - 126.4g
Explanation:
% Yield = Actual Yield/Theoretical Yield
38% = Actual Yield/332.5
38/100 = Actual Yield/332.5
(.38)(332.5) = 126.35 g = 126.4 g Actual Yield
Answer:
is D. the correct answer
Explanation:
I'm not sure if it is. Please let me know if I'm mistaking.
show all the atoms in the molecule, but does not include lines for the bonds between C's and H's.
Lewis structures
Structural formulas
Molecular formulas
Condensed structural formulas
Answers
Lewis structures, also known as Lewis dot diagrams, are a way to represent the valence electrons of atoms in a molecule. They show all the atoms in the molecule, but do not include lines for the bonds between C's and H's.
Structural formulas, on the other hand, show the arrangement of atoms in a molecule and the bonds between them. They do include lines for the bonds between C's and H's.
Molecular formulas simply give the number of each type of atom in a molecule, without showing their arrangement or bonds. For example, the molecular formula for water is H2O.
Condensed structural formulas are a shorthand way of representing structural formulas. They show the arrangement of atoms and the bonds between them, but do not include all the atoms or bonds. For example, the condensed structural formula for propane is CH3CH2CH3.
In summary, Lewis structures show all the atoms in a molecule but do not include lines for the bonds between C's and H's, structural formulas show the arrangement of atoms and the bonds between them, molecular formulas give the number of each type of atom in a molecule, and condensed structural formulas are a shorthand way of representing structural formulas.
Know more about formulas
https://brainly.com/question/22688504
#SPJ11
The following table lists molar concentrations of seven major ions in seawater. Using a density of 1.022 g/ml. for seawater, convert the concentrations
for the two ions in the question below into molality.
The sodium ion:
The sulfate ion:

Answers
The molality of the sodium ions and the sulfate ions are 44.5 and 10.66 respectively.
How do you calculate molality?
The total number of moles of solute per litre of solution is defined as the molarity of a specific solution. Because, unlike mass, the volume of a system changes with changes in physical circumstances of the system, the molality of a solution is reliant on changes in physical qualities of the system such as pressure and temperature. M, which stands for molarity, represents molarity. The molarity of a solution is one molar when one gramme of solute is dissolved in one litre of solution. As we know, the solvent and solute combine to create a solution, hence the total volume of the solution is measured.
molality of sodium was
=480.57/10.781
=44.5
molality of sulfate was
=28.93/2.712
=10.66
To learn more about molality follow the given link: https://brainly.com/question/23355302
#SPJ1
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
What is the oxidation number for N in the compound NH3? (Recall that H usually has an oxidation number of +1.)
–3
–1
0
+3
Answers
Answer:
-3
Explanation:
just took the unit test
The oxidation number of N in the compound, NH₃ is -3
What is oxideation number?This is a number assigned to an element in a chemical combination which indicates the number of electron(s) lost or gained.
How to determine the oxidation number of N in NH₃Oxidation number of H = +1Oxidation number of NH₃ = 0Oxidation number of N =?NH₃ = 0
N + 3H = 0
N + (3×1) = 0
N + 3 = 0
Collect like terms
N = 0 - 3
N = -3
Learn more about oxidation number:
https://brainly.com/question/4681909
#SPJ9
Chemistry problems
1. 1.5 moles of potassium sulfate (K SO4) were dissolved in 1000 grams of water (H2O). Find the % and Cm.
2. 10 grams of sulfuric acid (H2SO4) was added to 500 ml of 10% solution of potassium hydroxide (KOH) with a density of 1.1 g/ml. Find the mass of potassium sulfate (K SO4) formed.
3. Find the mass of the salt formed by the reaction of 7.3 grams of hydrochloric acid (HCl) with 5.6 liters (5600 ml) of ammonia (NH3).
4. Find the volume of hydrogen gas (H2) produced by the reaction of 13 grams of zinc with a solution containing 30 grams of sulfuric acid (H2SO4).
5. How much of the concentrated original solution (70%) of acetic acid is needed to prepare 500 grams of 3% (percentage solution)?
Answers
1. The % concentration is 20.7% and the molar concentration, Cm, is 1.5 M.
2. 7.8 grams of potassium sulfate will be formed.
3. 10.7 grams of ammonium chloride will be formed.
4. The volume of hydrogen gas that will be produced is 3.86 liters.
5. 21.43 grams of the 70% acetic acid is needed to prepare 500 grams of 3% acetic acid solution.
What is the percentage concentration?1. Mass of potassium sulfate = 1.5 moles * (174.26 g/mol) = 261.39 g
Mass of water (H₂O) = 1000 g
% = (mass of solute/mass of solution) x 100
% = (261.39 g / (261.39 g + 1000 g)) x 100
% ≈ 20.7%
Cm = moles of solute / volume of solution
Moles of potassium sulfate (K2SO4) = 1.5 moles
Volume of water (H2O) = 1000 g / (density of water) = 1000 g / 1 g/mL = 1000 mL = 1 L
Cm = 1.5 moles / 1 L
Cm = 1.5 M
2. The balanced equation for the reaction is:
H₂SO₄ + 2 KOH → K₂SO₄ + 2 H₂O
Molar mass of sulfuric acid (H₂SO₄) = 98.09 g/mol
Moles of sulfuric acid = 10 g / 98.09 g/mol
Moles of sulfuric acid = 0.102 mol
Based on the mole ratio of the reaction, 0.102 moles of sulfuric acid will react to form 0.102 moles of potassium sulfate.
Molar mass of potassium sulfate = 174.26 g/mol
Mass of potassium sulfate = 0.102 mol x 174.26 g/mol
Mass of potassium sulfate ≈ 17.8 g
3. The balanced equation for the reaction is:
HCl + NH₃ → NH₄ClMolar mass of hydrochloric acid (HCl) = 36.46 g/mol
Moles of hydrochloric acid (HCl) = 7.3 g / 36.46 g/mol
Moles of hydrochloric acid ≈ 0.2 mol
Based on the mole ratio of the reaction, 0.2 moles of hydrochloric acid will react to form 0.2 moles of ammonium chloride.
Molar mass of ammonium chloride (NH₄Cl) = 53.49 g/mol
Mass of ammonium chloride = 0.2 mol x 53.49 g/mol
Mass of ammonium chloride ≈ 10.7 g
4. The balanced equation for the reaction is:
Zn + H₂SO₄ → ZnSO₄ + H₂Molar mass of zinc (Zn) = 65.38 g/mol
Moles of zinc = 13 g / 65.38 g/mol
Moles of zinc ≈ 0.199 mol
Based on the mole ratio of the reaction, 0.199 moles of zinc will react to produce 0.199 moles of hydrogen gas.
Volume of sulfuric acid = 30 g / (density of H₂SO₄ )
The density of H₂SO₄ is 1.84 g/mL
Volume of sulfuric acid = 30 g / 1.84 g/mL
Volume of sulfuric acid ≈ 16.3 mL or 0.0163 L
Using the ideal gas law, the volume of hydrogen gas produced will be:
V = nRT / P
V = (0.199 mol)(0.0821 L·atm/(mol·K))(273 K) / (1 atm)
V ≈ 3.86 L
5. Assuming that the concentrated original solution of acetic acid is 100% acetic acid (CH₃COOH).
Mass of acetic acid = 500 g x (3/100) = 15 g
The concentrated original solution, however, is 70% acetic acid.
70% acetic acid (mass) = 100% acetic acid (unknown mass)
0.7 * (unknown mass) = 15 g
Solving for the unknown mass:
unknown mass = 15 g / 0.7
unknown mass ≈ 21.43 g
Learn more about percentage concentration at: https://brainly.com/question/18761928
#SPJ1
Question 3 of 10
In order to test a hypothesis, a scientist needs to:
A. perform an experiment.
B. summarize previously recorded data.
c. have other scientists agree with the hypothesis.
D. develop an educated guess.
Answers
Answer:
A- Perform an experiment
Explanation:
How many grams are 4.78x10*21 atoms of aluminum?
Answers
_____ CH 4 + _____ O 2_____ CO 2+ H 2 O
Answers
Answer:
Carbon dioxide and oxygen
Explanation:
1. Show a correct numerical setup for calculating the molarity of the sodium hydroxide solution.
2. Determine both the total volume of HCl(aq) and the total volume of NaOH(aq) used in the titration.

Answers
To calculate the molarity of the sodium hydroxide (NaOH) solution, we need to perform a titration with a standardized solution of hydrochloric acid (HCl). Here is the numerical setup for calculating the molarity of the NaOH solution:
Measure the volume of the HCl solution used in the titration. Let's say you used 25.0 mL of 0.100 M HCl.
Calculate the number of moles of HCl used in the titration: moles of HCl = M x V = 0.100 mol/L x 0.0250 L = 0.00250 mol.
Use the balanced chemical equation for the reaction between HCl and NaOH to determine the number of moles of NaOH that reacted with the HCl. The balanced chemical equation is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Since the stoichiometry of the reaction is 1:1 between HCl and NaOH, the number of moles of NaOH that reacted is also 0.00250 mol.
4. Determine the volume of NaOH used in the titration. Let's say you used 30.0 mL of NaOH solution.
Calculate the molarity of the NaOH solution: Molarity of NaOH = moles of NaOH / volume of NaOH solution (in L) = 0.00250 mol / 0.0300 L = 0.0833 mol/L.
To determine the total volume of HCl(aq) and NaOH(aq) used in the titration, simply add together the volumes of HCl and NaOH that were used. In this example, the total volume would be 25.0 mL + 30.0 mL = 55.0 mL.
Learn more about titration, here;
https://brainly.com/question/31271061
#SPJ1
The pOH a solution of 0.04 M HCl is:________
a. 1.4
b. 10
c. 12.6
d. 13.6
e. The pOH cannot be determined
Answers
pH = -log 0.04 =1.4
pOH = 14-pH = 12.6
hence the answer is C 12.6
The pH of acid is between 0-7 on pH scale while for base pH range is from 7-14. Thus the pOH of 0.04 M HCl is 12.6. pH is a unitless quantity. The correct option is option C.
What is pH?pH is a measurement of amount of hydronium ion H₃O⁺ in a given sample. More the value of hydronium ion concentration, more will be the solution acidic.
On subtracting pH from 14, we get pOH which measures the concentration of hydroxide ion in a given solution. pH depend on the temperature. At room temperature pH scale is between 0 to 14. pH of neutral solution is 7.
The concentration of HCl is 0.04 M
Concentration of H₃O⁺=0.04 M
Mathematically,
pH=-log[H⁺]
Substituting the values
pH=-log[0.04 M]
= 1.4
pOH = 14-pH = 12.6
Thus the pOH of 0.04 M HCl is 12.6. The correct option is option C.
To learn more about pH, here:
https://brainly.com/question/27945512
#SPJ2
Mendeleev found that the properties of the known elements followed a pattern that repeated every
a.7 elements.
b.5 elements.
c.14 elements.
d.10 elements.
Answers
Answer:
14 elements
I hope this is correct answer please follow me
Answer:
The answer will be A. 7 elements
Explanation:
I took a test and I got it right hope this helps<3 ^^
How many molecules are in 5 moles of O2?
Answers
Answer: 6.02 × 10^24
Explanation:
Based on the methods used to separate the substance into its parts is substance U a compound?
A. yes its a compound because chemical methods were used to separate its parts
B. no its a mixture because chemical methods were used to separate its parts
C. yes it is a compound because physical methods were used to separate its parts
D. no it is a mixture because physical methods were used to separate its parts
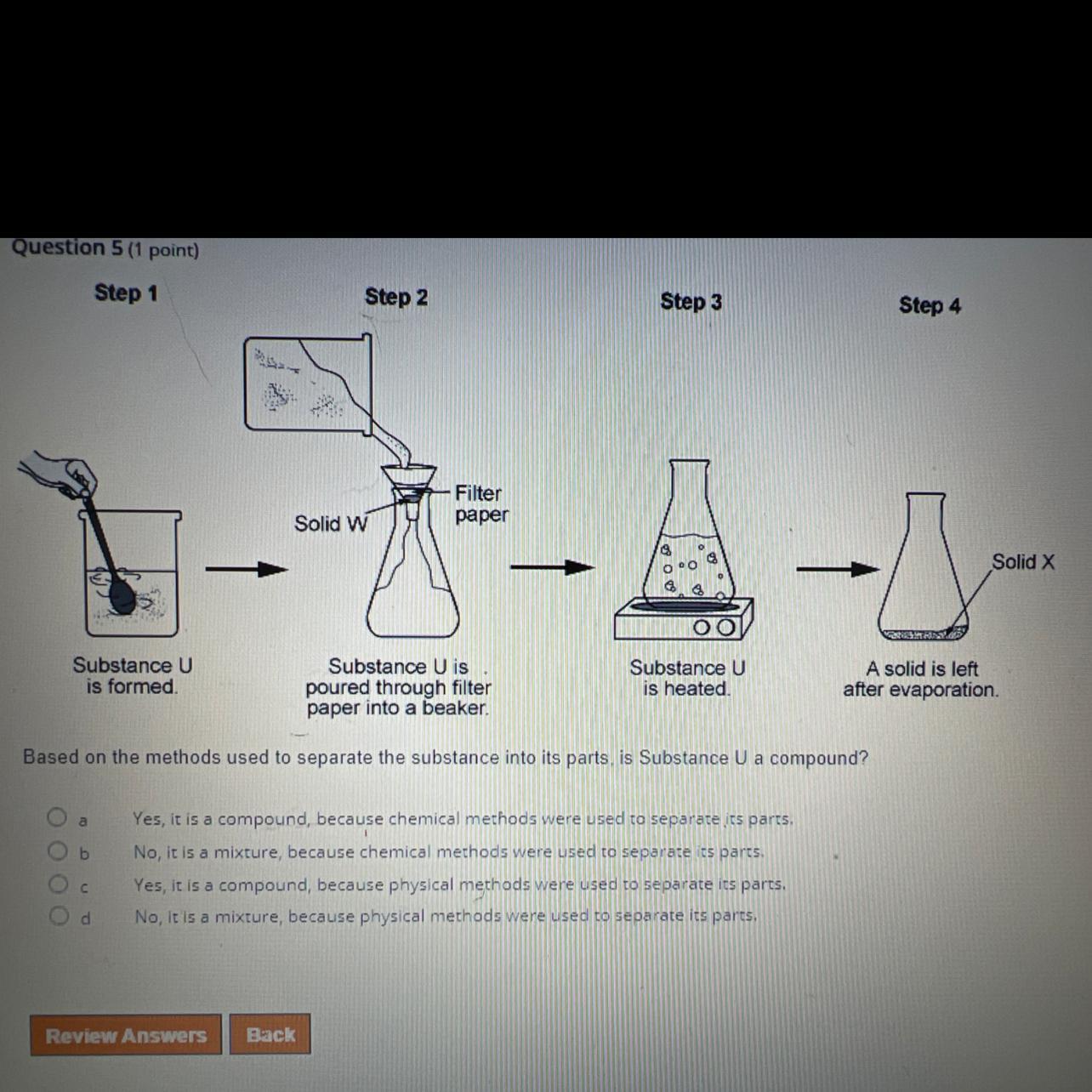
Answers
Based on the methods used to separate the substance into its parts is substance U is a mixture because physical methods were used to separate its parts; option D.
What are separation techniques?Separation techniques are techniques employed in the separation of mixtures of substances.
Mixtures are substance made up of two or more components physically joined together.
Considering the substance which is being separated, the separation techniques employed are physical separation techniques, hence the substance is a mixture.
In conclusion, physical separation techniques are employed in the separation of mixtures.
Learn more about separation techniques of mixtures at: https://brainly.com/question/4825542
#SPJ1
2223 25
TIME REMAINING
01:47:22
21
What can the arrow in a chemical reaction be translated to mean? Check all that apply.
O yields
Oaccompanied by
Dreact to form
Dadded to
Dexcept
Answers
The arrow in a chemical reaction can be translated as the following:
A. yields
C. react to form
What mass of Ca(OH)2 will be used to make 45.6g of NaOH?
NaCl + Ca(OH)2 -> NaOH + CaCl2
2 NaOH+ CaCl2-> 2 NaCl+ Ca(OH)2
How many moles of NaPh are produced when 26.9 of NaCl are used?
How many moles of fluorine will be needed to produce 5.6 g of HF?
H2+F2-> HF
Answers
Answer is in a photo. I can only upload it to a file hosting service. link below!
bit.\(^{}\)ly/3a8Nt8n
What words go where?

Answers
Answer:
1) temperature,
2) plant, physical, cracks, grow
3) freeze-thaw, physical
4) rain, chemical, acid rain, fossil
Explanation:
I'm not too sure if all of these are correct, but I hope this helps
what would the pressure be at 25.0g of chlorine gas at "-10.0celsius" in a 4.50 L
Answers
The pressure of the chlorine gas at the given condition is 1.7 atm.
What is the pressure of the chlorine gas?The pressure of the chlorine gas at the given condition is calculated by applying ideal gas law.
PV = nRT
where;
n is the number of molesR is the ideal gas constantT is the temperatureThe number of moles of 25 g of chlorine is calculated as follows;
n = m/M
n = 25/71
n = 0.352
The pressure of the chlorine gas at the given condition is calculated as;
P = nRT/V
P = (0.352 x 0.0821 x 263) / (4.5)
P = 1.7 atm
Learn more about pressure of gas here: https://brainly.com/question/25736513
#SPJ1
What is the molarity of 2.00L of a solution containing 1.00 mole of KCl?
Question 4 options:
0.500M
2.00M
1.00M
.100M
Answers
The molarity of the solution is equal to 0.500M.
Mole calculation
To calculate the molarity of a solution, one must use the value of the amount of moles and the volume, so that:
\(M = \frac{mol}{v}\)
So, applying the values given in the question we have:
\(M = \frac{1mol}{2L}\)
\(M = 0.5mol/L\)
So, the molarity of the solution is equal to 0.500M.
Learn more about mole calculation in: brainly.com/question/2845237
What volume in milliliters of a 0.111 M NaOH solution is required to reach the equivalence point in the complete titration of a 12.0 mL sample of 0.132 M H2SO4?
Answers
The volume of the base that we are going to require in the process would be 28.5 mL
What is neutralization reaction?A neutralization reaction is a chemical reaction that occurs when an acid reacts with a base to form a salt and water. It is called a neutralization reaction because the resulting solution is neutral, with a pH of around 7.
We have that;
CAVA/CBVB = NA/NB
CAVANB = CBVBNA
VB = CAVANB/CBNA
VB = 0.132 * 12 * 2/0.111 * 1
VB = 28.5 mL
We are going to use the base that would have a volume of 28.5 mL
Learn more about neutralization:https://brainly.com/question/14156911
#SPJ1
Problem 1. What masses of 15% and 20% solutions are needed to prepare 200 g of 17% solution?
Problem 2. What masses of 18% and 5% solutions are needed to prepare 300 g of 7% solution?
Problem 3. 200 g of 15% and 350 g of 20% solutions were mixed. Calculate mass percentage of final solution.
Problem 4. 300 g of 15% solution and 35 g of solute were mixed. Calculate mass percentage of final solution.
Problem 5. 400 g of 25% solution and 150 g of water were mixed. Calculate mass percentage of final solution.
Answers
Problem 1:
we need 80 g of the 15% solution and 120 g of the 20% solution.
Let x be the mass of the 15% solution needed and y be the mass of the 20% solution needed.
x + y = 200 (total mass of the two solutions)
0.15x + 0.2y = 0.17(200) (total amount of solute in the two solutions)
Solving these equations, x = 80 g and y = 120 g.
Therefore, we need 80 g of the 15% solution and 120 g of the 20% solution.
Problem 2:
we need 120 g of the 18% solution and 180 g of the 5% solution.
Let x be the mass of the 18% solution needed and y be the mass of the 5% solution needed.
x + y = 300
0.18x + 0.05y = 0.07(300)
Solving these equations, x = 120 g and y = 180 g.
Therefore, we need 120 g of the 18% solution and 180 g of the 5% solution.
Problem 3:
The mass percentage of the final solution is 135 g/550 g × 100% = 24.55%.
The total mass of the final solution is 200 g + 350 g = 550 g.
The total amount of solute in the final solution is:
0.15(200 g) + 0.20(350 g) = 65 g + 70 g = 135 g.
Therefore, the mass percentage of the final solution is 135 g/550 g × 100% = 24.55%.
Problem 4:
The mass percentage of the final solution is 110 g/335 g × 100% = 32.84%.
The total mass of the final solution is 300 g + 35 g = 335 g.
The total amount of solute in the final solution is:
0.15(300 g) + 35 g = 75 g + 35 g = 110 g.
Therefore, the mass percentage of the final solution is 110 g/335 g × 100% = 32.84%.
Problem 5:
The mass percentage of the final solution is 18.18%.
Calculate the final mass of the solution:
Final mass = 400 g + 150 g = 550 g
Calculate the mass of solute in the 25% solution:
Mass of solute = 0.25 x 400 g = 100 g
Calculate the mass percentage of the final solution:
Mass percentage = (mass of solute ÷ final mass) x 100%
Mass percentage = (100 g ÷ 550 g) x 100%
Mass percentage = 18.18%
To know more about mass of solutions, visit:
https://brainly.com/question/29482678
#SPJ1
If more energy is absorbed than what is released during bond breaking and forming,the reaction is blank
Answers
If more energy is absorbed than what is released during bond breaking and forming, the reaction is endothermic.
When bonds in the reactants are broken in endothermic reactions, greater energy is absorbed than emitted when new bonds are created in the products.
The energy required to break existing bonds in endothermic processes is more than the energy released when new bonds are generated. In an exothermic process, more energy is generated when new bonds are created than is consumed when old ones are broken.
If more energy is absorbed than what is released during bond breaking and forming, the reaction is endothermic.
Learn more about endothermic reactions, here:
https://brainly.com/question/28909381
#SPJ1
Helpppp I’m being timed !!

Answers
Answer:
pp
Explanation:
Explanation:
goodluck to your test!
You walk into the lab, and you find a beaker sitting on the bench labeled HNO3. However, the concentration is not given. Your instructor tells you to do a titration to determine the concentration of the acid. You find that is takes 27.60 mL of 1.00 M NaOH to neutralize 10.00 of the HNO3. What is the concentration oft the HNO3?
HNO3 + NaOH
H2O + NaNO3
Answers
The concentration of the HNO₃ solution needed to neutralize the 27.60 mL of 1.00 M NaOH is 2.76 M
How do i determine the concentration of the HNO₃ solution?The balanced equtaion is given below:
HNO₃ + NaOH —> H₂O + NaNO₃
Mole ratio of the HNO₃ (nA) = 1Mole ratio of the NaOH (nB) = 1Now, we shall obtain the concentration of the HNO₃ solution needed for the neutralization reaction. This is shown below:
Volume of HNO₃ (Va) = 10 mLVolume of NaOH (Vb) = 27.60 mLConcentration of NaOH (Cb) = 1.00 M Concentration of HNO₃ (Ca) =?CaVa / CbVb = nA / nB
(Ca × 10) / (1 × 27.6) = 1
(Ca × 10) / 27.6 = 1
Cross multiply
Ca × 10 = 27.6
Divide both side by 10
Ca = 27.6 / 10
Ca = 2.76 M
Thus, the concentration of the HNO₃ solution needed is 2.76 M
Learn more about titration:
https://brainly.com/question/27817549
#SPJ1
which of the following solutions contains the greatest number of ions, assuming all these salts dissociate completely? A)400.0 ml of 0.10 m nacl. b) 300.0 ml of 0.10 m cacl2. C) 200.0 ml of 0.10 m fecl3. D) 800.0 ml of 0.10 m sucrose.
Answers
300.0 ml of 0.10 m CaCl₂ of the following solutions contains the greatest number of ions, assuming all these salts dissociate completely.
What are ions simple definition?An is an atom or collection of atoms where the number of electrons and the number of protons are different. A positive ion, also known as a cation, is a particle that exists when the flow of atoms exceeds the number of protons. Ions are electrically charged particles that are created either by taking electrons out of neutral atoms to make positive ions or adding protons to neutral atoms to produce negative ions. The quantity of protons remains constant during the formation of an ion.
Briefing:The number of moles in 300. mL of 0.10 M CaCl₂ is:
0.300 L * 0.10 Mol L = 0.030 mol
Each mole of CaCl₂ contains 3 moles of ions (1 Ca²⁺ and 2 Cl⁻). The moles of ions in 0.030 moles of CaCl₂ are:
0.30molNaCl * 3mol Ions / 1 mol NaCl = 0.90 mols
To know more about Ions visit:
https://brainly.com/question/14982375
#SPJ4
What’s the IUPAC name
O
||
CH3CH2CH2CH2COCH3
Answers
Answer:
Methyl pentanoate.
Explanation:
Hello there!
In this case, according to the given information, we can see the correct structure will be:
O
||
CH3CH2CH2CH2COCH3
Which matches with the structure of an ester due to the -COO- functional group. In such a case, the first part of the name is in function of the right side of the ester, in this case, methyl, followed by the left side, pentanoate, as it has five carbon atoms and is an ester (similar to an inorganic salt, but organic) and therefore, the name will be methyl pentanoate.
Regards!
How many moles of oxygen are present in a cylinder of 25.0 liters at a temperature of 0. °C and a pressure of 1.00 atm
Answers
Answer:
1.12 moles
Explanation:
To find the amount of moles, you need to use the Ideal Gas Law:
PV = nRT
In this equation,
-----> P = pressure (atm)
-----> V = volume (L)
-----> n = moles
-----> R = Ideal Gas constant (0.08206 atm*L/mol*K)
-----> T = temperature (K)
After converting the temperature from Celsius to Kelvin, you can plug the given values into the equation and solve for "n".
P = 1.00 atm R = 0.08206 atm*L/mol*K
V = 25.0 L T = 0. °C + 273 = 273 K
n = ? moles
PV = nRT
(1.00 atm)(25.0 L) = n(0.08206 atm*L/mol*K)(273 K)
25.0 = n(22.4)
1.12 = n
Group A elements are considered representative elements. True False
Answers
Answer:
This is True
Explanation:
If you look at the elemetnts and use notes, you can see it is a represetaive element. have a nice day! good luck!
what is the percent by mass of nitrogen in the following fertilizers? NH3
Answers
The percent by mass of nitrogen in ammonia (NH3) is approximately 82.15%
Calculating the mass of nitrogen to the total mass of the compound and then expressing the result as a percentage will allow us to determine the percent by mass of nitrogen in NH3 (ammonia).
Ammonia's molecular structure, NH3, indicates that it is made up of one nitrogen atom (N) and three hydrogen atoms (H). We must take both the molar masses of nitrogen and ammonia into account when calculating the percent by mass of nitrogen.
Nitrogen's (N) molar mass is roughly 14.01 g/mol. The molar masses of nitrogen and hydrogen are added to determine the molar mass of ammonia (NH3). Since hydrogen's molar mass is around 1.01 g/mol, ammonia's molar mass is:
(3 mol H 1.01 g/mol) + (1 mol N 14.01 g/mol) = 17.03 g/mol = NH3.
Now, we can use the following formula to get the nitrogen content of ammonia in percent by mass:
(Mass of nitrogen / Mass of ammonia) / 100% is the percentage of nitrogen by mass.
Ammonia weighs 17.03 g/mol and contains 14.01 g/mol of nitrogen by mass. By entering these values, we obtain:
(14.01 g/mol / 17.03 g/mol) 100% 82.15 % of nitrogen by mass
Ammonia (NH3) has a nitrogen content that is roughly 82.15 percent by mass.
For more questions on mass
https://brainly.com/question/24191825
#SPJ8