What is the empirical formula for C10h22
Answers
The empirical formula is
C5H11
Explanation:
We have decane, the alkane with a chemical formula of
C10H22
.
The empirical formula of a substance has the smallest ratio between the elements its composed of.
Here, we got the carbon to hydrogen ratio as
10:22
. So, we need to find the greatest common factor between the two numbers.
10=2*5
22=2*11
As you can see here, the greatest common factor between the two numbers is
2
, and so we divide both numbers by
2
.
10/2=5
22/2=11
And so, the new formula becomes
C5H11
Related Questions
The area of an object is calculated from experimental data to be 24.6623 cm2. The ± absolute error in the area was determined to be ± 0.6 cm2. The area should be reported, in cm 2 , as A. 25 B. 24.7 C. 24.66 D. 24.6623 E. 24.662
Answers
we should take out from point
A polluted stream with flow rate Qs=10.0 m^3/s and pollution concentration Cs=20.0mg/L flows into a lake of volume V=12×106 m3. In addition, a wastewater outlet discharges Qw=3.0 m3/s with pollution concentration cw = 229mg/L into the lake. The pollution has a first order decay reaction rate coefficient of K=0.14 per day. An outgoing stream maintains constant water level in the lake. Assume complete mixing in the lake, and no evaporation or other water losses or gains. What is the steady state concentration of the pollutant in the lake? (Answer units: mg/L)
Answers
The steady-state concentration of the pollutant in the lake is approximately 0.000528 mg/L.
To find the steady-state concentration of the pollutant in the lake, we need to consider the inflow and outflow rates of the pollutant and the decay reaction rate.
The total inflow rate of the pollutant is given by:
Qin = Qs * Cs + Qw * Cw
The total outflow rate is equal to the flow rate out of the lake, which is the same as the inflow rate:
Qout = Qin
The change in the pollutant concentration in the lake over time can be expressed by the following equation:
dC/dt = (Qin - Qout)/V - K * C
At steady state, the change in concentration is zero (dC/dt = 0). Rearranging the equation, we have:
(Qin - Qout)/V - K * C = 0
Substituting the given values:
(10.0 m^3/s * 20.0 mg/L + 3.0 m^3/s * 229 mg/L)/12×10^6 m^3 - 0.14 per day * C = 0
Simplifying the equation:
(200.0 mg/s + 687.0 mg/s)/12×10^6 m^3 - 0.14 per day * C = 0
887.0 mg/s / 12×10^6 m^3 - 0.14 per day * C = 0
0.0000739 mg/L/s - 0.14 per day * C = 0
0.0000739 mg/L/s = 0.14 per day * C
Solving for C, the steady-state concentration:
C = (0.0000739 mg/L/s) / (0.14 per day)
C ≈ 0.000528 mg/L
Learn more about concentration visit:
brainly.com/question/31102877
#SPJ11
need a top pick for another slide to talk about crystals
Answers
Answer:
tigers eyes
Explanation:
In a new compound, it is found that the central carbon atom is sp2 hybridized. This implies that.
Answers
In a new compound, it is found that the central carbon atom is sp2 hybridized, this implies that carbon is also involved in a pi bond.
Hybridization of Carbon-Carbon is one of the important and most common chemical elements that are essential for organic connections.
A carbon atom is sp2 hybridized when bonding takes place between 1 s- orbital with two p orbitals. There is a formation of two single bonds and one double bond between three atoms. The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds.
In sp2 hybridization, one s orbital and two p orbitals hybridize to form three sp2 orbitals, each consisting of 33% s character and 67% p character.
Therefore when the central carbon atom is sp2 hybridization then there must be carbon involves in a pi bond.
To know more about hybridization refer to the link given below:
https://brainly.com/question/22765530
#SPJ4
I just need help with question 10.??? Please ASAP
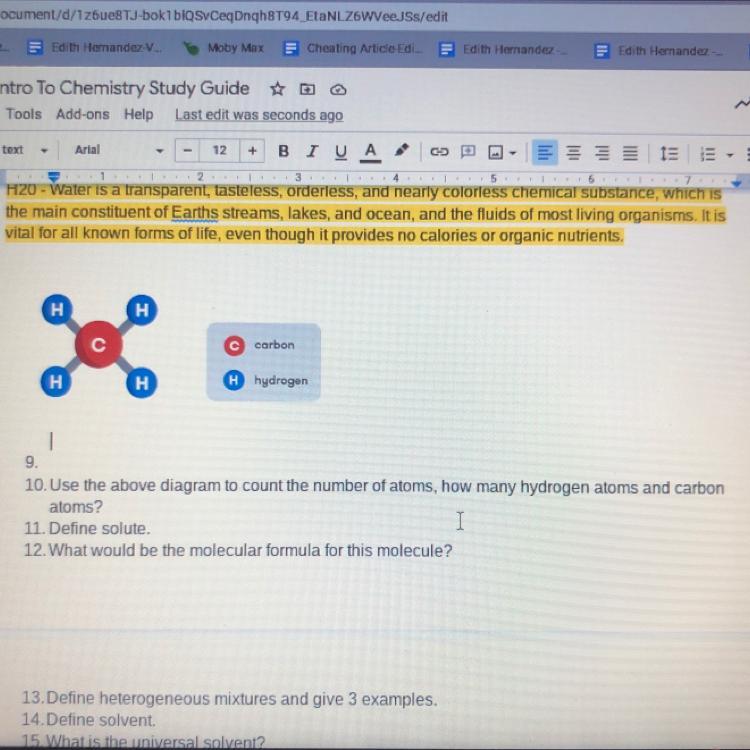
Answers
Answer:
4 hydrogen / 1 Carbon
Just look at the diagram
Sulfuric acid was once produced through the reaction of sulfur trioxide with water. Sulfur trioxide can form through the reaction of sulfur dioxide and oxygen gas. When nitrogen monoxide gas is added to the system, the reaction speeds up significantly because it proceeds through the following steps: First: 2 upper N upper O (g) plus upper O subscript 2 (g) right arrow 2 Upper N upper O subscript 2 (g). Second: 2 upper N upper O subscript 2 (g) plus 2 upper S upper O subscript 2 right arrow 2 Upper N upper o (g) plus 2 upper s upper o subscript 3 (g). Identify the catalyst in this reaction, explain how you know it is the catalyst, and describe how it increases the rate of the reaction.
Answers
Answer:
NO is the catalyst; it provides a reaction pathway with a lower activation energy
Explanation:
1. Identify the catalyst
A catalyst is a species that is present at the beginning of a reaction and reappears at the end.
It does not appear in the overall equation.
Let's apply these concepts to your mechanism:
First: 2NO(g) + O₂(g) ⟶ 2NO₂(g)
Second: 2NO₂(g) +2SO₂(g) ⟶ 2NO(g) + 2SO₃(g)
Overall: O₂(g) + 2SO₂ ⟶ 2O₂
We see that NO is present at the beginning of the first step. It reacts and then re-forms in the second step. It does not appear in the final equation.
NO is the catalyst.
2. Explain the action of the catalyst
A catalyst acts by providing an alternate pathway with a lower activation energy.
The direct reaction of SO₂ with oxygen to form SO₃ has a high activation energy.
NO reacts with the oxygen to form an intermediate (NO₂) that then reacts with the SO₂ to form SO₃. Both steps have lower activation energies, so the reaction is faster.
Answer:
NO is the catalyst. NO is the catalyst because it increases the rate of the reaction but is not consumed during the reaction. NO increases the rate of the reaction by lowering the activation energy. The reaction of NO with O2 provides an alternative reaction pathway with a lower activation energy.
Explanation: edge 2020
How can you separate sand and sugar?
Answers
Answer:
1)Filtration to remove sand
2)Evaporation to obtain sugar
Explanation:
Add water to the mixture and mix,sugar will dissolve.
Filter out the sand.
Evaporate the remaining solution to obtain the sugar.
What is the concentration of hydronium ion in a 0.121 M HCl solution?
A) 1.0 M
B) < 0.121 M
C) 0.121 M
D) not enough information
Answers
Answer:
C) 0.121 M
Explanation:
HCl + H₂O = H₃O⁺ + OH⁻
.121M .121M
HCl is a strong acid . It will dissociate almost 100 % so the concentration of acid and hydronium ion formed will be equal . It is to be noted that hydronium ion is formed due to association of H⁺ and H₂O . H⁺ is formed due to ionisation of HCl .
So concentrtion of hydronium ion ( H₃O⁺ ) will be .121 M.
If 45.6 g of liquid water were produced in this reaction, how many grams of sodium hydroxide were consumed?
Answers
50.68 grams of sodium hydroxide were consumed in the reaction.
Since the balanced chemical equation for this reaction is:
2 Na + 2 H2O -> 2 NaOH + H2
We can see that for every 2 moles of Na used, 2 moles of NaOH are produced, and 1 mole of H2O is produced. Therefore, the moles of NaOH produced in the reaction can be calculated by dividing the moles of H2O produced by 1.
First, let's calculate the moles of H2O produced:
m(H2O) = 45.6 g / 18.015 g/mol = 2.534 mol
Since the stoichiometric ratio of NaOH to H2O is 2:1, we know that the number of moles of NaOH produced is half the number of moles of H2O produced:
n(NaOH) = 1/2 x n(H2O) = 1.267 mol
Now we can use the molar mass of NaOH to calculate the mass of NaOH consumed:
m(NaOH) = n(NaOH) x MM(NaOH) = 1.267 mol x 40.00 g/mol = 50.68 g
For more such questions on sodium hydroxide visit:
https://brainly.com/question/25597694
#SPJ11
4 pt
Question 6
(05.06 LC)
The actual yield of a product in a reaction was measured as 2.80 g. If the theoretical yield of the
product for the reaction is 3.12 g, what is the percentage yield of the product? (4 points)
82.7%
85.2%
87.3%
89.7%
Answers
89.74% is the percentage yield of the product. Hence, option D is correct.
What is the meaning of percentage?The percentage can be calculated by dividing the value by the total value and then multiplying the result by 100.
Given data:
Actual yield of product = 2.80 g
Theoretical yield = 3.12 g
Percent yield = ?
Formula:
Percent yield = \(\frac{\;actual \;yield \;X \;100}{theoretical \;yield}\)
by putting values in the formula,
Percent yield =\(\frac{2.80 g X 100}{ 3.12 g}\)
Percent yield = 89.74%
Hence, option D is correct.
Learn more about percentages here:
https://brainly.com/question/20758645
#SPJ1
calculate the work (kj) done during a reaction in which the internal volume contracts from 90 l to 14 l against an outside pressure of 7 atm
Answers
Sure, I'd be happy to help! To calculate the work done during a reaction, we use the formula:work = -PΔV
Where P is the pressure, ΔV is the change in volume, and the negative sign indicates that work is being done on the system (since the volume is contracting).
In this case, the outside pressure is given as 7 atm, and the internal volume contracts from 90 L to 14 L. So we can calculate ΔV as
(Note that the units are J, not kJ - I assume this was a typo in the original question.)
So the work done during this reaction is 532 J. I hope this helps! Let me know if you have any other questions.
Hello! I'd be happy to help you with your question. To calculate the work done during a reaction with a change in volume and given outside pressure, you can use the formula:
Work (W) = -P * ΔV- P is the outside pressure (7 atm in this case)
- ΔV is the change in volume, calculated as V_final - V_initial (14 L - 90 L in this case)
Step 1: Calculate the change in volume (Δ
Before we proceed with this calculation, it's important to note that we need to convert the units to kJ. The conversion factor is 1 L·atm = 0.1013 kJ. So, we will multiply our result by this conversion facto
W = (-7 * -76) * 0.1013 kJ
W = 532 * 0.1013 kJ
W = 53.8916 kJ
Therefore, the work done during the reaction is approximately 53.89 kJ.
learn more about
https://brainly.com/question/28984750
#SPJ11
is sugar water a substance or mixture?
Answers
Answer: mixture
Explanation: Answer my own question on my page please.
Answer: A mixture.
Explanation: A substance has to do with the chemical composition changing, so if you just dissolve sugar into water nothing changes in either’s chemical composition.
fill in the blanks with the words given below-
[Atoms, homogeneous, metals, true, saturated, homogeneous, colloidal, compounds, lustrous]
1.An element which are sonorous are called................
2.An element is made up of only one kind of ....................
3.Alloys are ............................. mixtures.
4.Elements chemically combines in fixed proportion to form ........................
5. Metals are................................... and can be polished.
6. a solution in which no more solute can be dissolved is called a .................... solution.
7. Milk is a .............. solution but vinegar is a .................. solution.
8. A solution is a ................... mixture.
pls help, could not get these answers
Answers
Answer:
1. Metal.
2. Atom.
3. Homogeneous
4. Compounds.
5. Lustrous
6. Saturated.
7. Colloidal; true.
8. Homogeneous.
Explanation:
1. An element which are sonorous are called metal.
2. An element is made up of only one kind of atom.
3. Alloys are homogeneous mixtures.
4. Elements chemically combines in fixed proportion to form compounds.
5. Metals are lustrous and can be polished.
6. A solution in which no more solute can be dissolved is called a saturated solution.
7. Milk is a colloidal solution but vinegar is a true solution.
8. A solution is a homogeneous mixture.
Help me for brainlest and 100 point

Answers
Answer: Stamen, the male reproductive part of a flower. In all but a few extant angiosperms, the stamen consists of a long slender stalk, the filament, with a two-lobed anther at the tip. The anther consists of four saclike structures (microsporangia) that produce pollen for pollination. The main flower parts are the male part called the stamen and the female part called the pistil. The stamen has two parts: anthers and filaments. The anthers carry the pollen. The four main parts of a flower are the petals, sepals, stamen, and carpel (sometimes known as a pistil). If a flower has all four of these key parts, it is considered to be a complete flower.
Explanation:
______ is a way to slow down the reactions that spoil milk

Answers
Answer:
b. keeping milk cold
Explanation:
The cool temperature in the fridge will stop it from going to any further reactions so it won't get spoilt
d. Keeping milk warm can help in slowing down of the reaction that can spoil milk.
Pasteurization can be defined as the heat treatment process in which the heat is provided to a liquid for example milk to prevent the growth of microbes which are least resistant to the heat. This process destroy the microbes, their spores, and also prevent their colonization.This process is useful in preventing soreness of milk and extends its shelf life. Keeping milk in cold container, in a cold temperature, or away from other sources of food will not help in slowing down the reactions that spoil milk.Hence, d. Keeping milk warm can help in slowing down of the reaction that can spoil milk.
Learn more about milk:
https://brainly.com/question/22512463
How do the number of particles present affect the freezing point of water?
Answers
Answer:
the greater the concentration of particles the lower the freezing point will be.
What is the meaning of this painting

Answers
Answer: things arnent always as they seem
Explanation:
why was it so important that the electron was smaller than the atom?
Answers
Answer:
because electron is a negetively charge particle that orbits the outer part of atom and despite being small it is a strong proton.
Explanation:
Why do animals eat other organisms? ILL MARK BRAINLIEST
Animals cannot produce their own energy without consuming other organisms.
Animals eat other organisms to get water.
Animals eat other organisms to provide food for decomposers.
Animals are part of the chain that prevents the plant population from disappearing.
Why are birds considered as consumers?
Birds eat decomposers.
Birds eat plants.
Birds eat other organisms for energy.
Birds make their own energy by absorbing chemicals.
Why do you see some animals in different food chains?
Some animals consume more than one type of organism.
Some animals can jump from one food chain to another depending on the season.
Some animals are a food source for different types of decomposers.
Some animals require to eat more because they are bigger.
Why is it possible that some animals may live side by side?
Some animals may consume the same type of plants.
There is a limited amount of space found in ecosystems.
Some animals may not be in the same food chain.
There is a limited amount of water supply in some ecosystems.
Choose the best statement that explains why do organisms need energy?
Energy is necessary only for hunting.
Energy is necessary for cells to function.
Energy is necessary only to make egg and sperm cells.
Energy is only needed during the daytime.
Answers
This are the required answers based on the food chain and ecosystem.
If you have any doubts regarding on my answer. Feel free to share the doubts with me in comments section :D
Answer:Animals eat other organisms — Animals cannot produce their own energy without consuming other organisms.
Birds considered as consumers — Birds eat plants.
We see some animals in different food chain — because some animals consume more than one type of organism.
Some animals may live side by side — There is a limited amount of space found in ecosystems.
The best statement that explains why do organisms need energy — Energy is necessary for cells to function.
This are the required answers based on the food chain and ecosystem.
If you have any doubts regarding on my answer. Feel free to share the doubts with me in comments section :D
Explanation:
Logan demonstrates to the class how mass must be conserved in every chemical reaction.
He measures the mass of hydrochloric acid and a magnesium strip separately. He then places the magnesium strip into the acid and bubbles form as the magnesium
seems to disappear. The combined mass afterward is less than the original.
Hydrochloric Acid + Magnesium
How could Logan explain this lower mass?
Answers
Answer:
Logan could explain the lower mass by explaining that the reaction between hydrochloric acid and magnesium produces hydrogen gas, which escapes and therefore is not included in the final combined mass measurement. The reaction is:HCl + Mg → MgCl2 + H2Since the hydrogen gas has mass, its escape from the reaction vessel explains the decrease in the combined mass of the reactants after the reaction.
It is beneficial for plants to have their seeds dispersed to reduce competition. Explain how a bird eating fruit is an example of mutualism.
Answers
Answer:
It both benefits the bird by providing sustenance and the plant by possibly spreading seeds through fecal matter.
Explanation:
OSTOICHIOMETRY
Using molarity to find solute moles and solution volume
A chemist adds 440.0 mL of a 1.46M barium acetate
added to the flask. Round your answer to 3 significant digits.
mol
be (Ba(C₂H₂O₂),) solution to a reaction flask, Calculate the millimoles of barium acetate the chemist has
X
Calculator
542400
Maribel V
do
Answers
The chemist has 642.4 millimoles of barium acetate in the solution.
To calculate the millimoles of barium acetate (Ba(C₂H₃O₂)₂) in the solution, we can use the formula:
moles = molarity × volume (in liters)
First, let's convert the volume from milliliters (mL) to liters (L):
440.0 mL ÷ 1000 = 0.440 L
Now we can substitute the given values into the formula:
moles = 1.46 M × 0.440 L
moles = 0.6424 mol (rounded to 4 decimal places)
To convert the moles to millimoles, we multiply by 1000:
millimoles = 0.6424 mol × 1000
millimoles = 642.4 mmol (rounded to 3 significant digits)
Therefore, the chemist has 642.4 millimoles of barium acetate in the solution.
It's important to note that the molarity (M) represents the number of moles of solute per liter of solution. By multiplying the molarity by the volume in liters, we can find the number of moles of solute. To convert moles to millimoles, we multiply by 1000. The result represents the millimoles of barium acetate present in the given volume of solution.
For more such questions on barium acetate visit:
https://brainly.com/question/15304103
#SPJ8
Which of the following describes what happens in a chemical reaction?
O A. Molecules change from one phase to another.
B. Molecules dissolved in solution are separated again.
O c. Chemical bonds are broken, and new ones are formed.
O D. Molecules mix without rearranging atoms.
Answers
Answer:
c. Chemical bonds are broken, and new ones are formed.
Explanation:
Hope this helps. :)
In a chemical reaction, chemical bonds are broken, and new ones are formed.
What is a chemical reaction?Chemical reactions are defined as reactions which occur when a substance combines with another substance to form a new substance.Alternatively, when a substance breaks down or decomposes to give new substances it is also considered to be a chemical reaction.
There are several characteristics of chemical reactions like change in color, change in state , change in odor and change in composition . During chemical reaction there is also formation of precipitate an insoluble mass of substance or even evolution of gases.
There are three types of chemical reactions:
1) inorganic reactions
2)organic reactions
3) biochemical reactions
During chemical reactions atoms are rearranged and changes are accompanied by an energy change as new substances are formed.
Learn more about chemical reactions,here:
https://brainly.com/question/29762834
#SPJ2
1
How many atoms of carbon would be present in a molecule of CCl4?
A
5
B
1
С
2
D 4
Answers
Answer:
There are 5! goodluck,
Explanation:
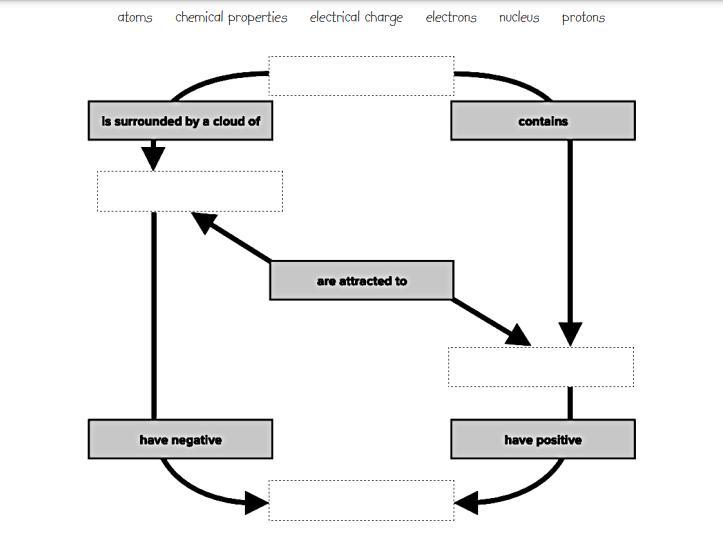
Complete the map by filling in the missing concepts. Atoms brain pop
Answers
Nucleus is surrounded by a cloud of electrons.
Nucleus contains protons.
Protons are attracted to electrons.
Electrons have negative electrical charge.
Protons have positive electrical charge.
What are the particles present in an atom?An atom is the smallest particle of an element that can take part in a chemical reaction.
Atoms of elements are composed of three sub-atomic particles, namely:
Protons -found in the nucleus and are positively chargedElectrons - surround the nucleus and are negatively chargedNeutrons - found in the nucleus and have a neutral charge.Learn more about atomic particles at: https://brainly.com/question/28763045
#SPJ1
T or F. Most nonmetals are poor conductors of heat.
Plz answer correctly.
Answers
Answer:
True
Explanation:
_H2SO4 + _Ca(OH)2 —> _CaSO4 + _H2O balance it
Answers
Answer:
2H2SO4 + 2Ca(OH)2 —> 2CaSO4 + 4H2O
when an ionic compound such as sodium chloride (nacl) is placed in water, the component atoms of the nacl crystal dissociate into individual sodium ions (na ) and chloride ions (cl-). in contrast, the atoms of covalently bonded molecules (e.g., glucose, sucrose, glycerol) do not generally dissociate when placed in aqueous solution. which of the following solutions would be expected to contain the greatest number of solute particles (molecules or ions)? when an ionic compound such as sodium chloride (nacl) is placed in water, the component atoms of the nacl crystal dissociate into individual sodium ions (na ) and chloride ions (cl-). in contrast, the atoms of covalently bonded molecules (e.g., glucose, sucrose, glycerol) do not generally dissociate when placed in aqueous solution. which of the following solutions would be expected to contain the greatest number of solute particles (molecules or ions)? 1 liter of 1.0 m glucose 1 liter of 0.5 m nacl 1 liter of 1.0 m nacl and 1 liter of 1.0 m glucose will contain equal numbers of solute particles. 1 liter of 1.0 m nacl
Answers
The solution that would be expected to contain the greatest number of solute particles is 1 liter of 1.0 m NaCl. Option C is the correct answer.
Ionic compounds are chemical compounds composed of ions held together by electrostatic forces of attraction between oppositely charged ions.
When an ionic compound such as NaCl is placed in water, the component atoms of the NaCl crystal dissociate into individual sodium ions (\(\rm Na^+\)) and chloride ions (\(\rm Cl^-\)).
To determine which of the solutions would be expected to contain the greatest number of solute particles, we need to consider the number of solute particles produced by each molecule of the solute.
NaCl dissociates in water to produce two solute particles (\(\rm Na^+\)and \(\rm Cl^-\)), while glucose does not dissociate and therefore produces only one solute particle. Therefore, 1 liter of 1.0 m NaCl would contain twice as many solute particles as 1 liter of 1.0 m glucose.
In conclusion, 1 liter of 1.0 m NaCl solution would be expected to contain the greatest number of solute particles. The correct answer is option C.
Learn more about Ionic compounds here:
https://brainly.com/question/30420333
#SPJ4
The given question is in inappropriate manner. The correct question is:
When an ionic compound such as sodium chloride (NaCl) is placed in water, the component atoms of the NaCl crystal dissociate into individual sodium ions and chloride ions. In contrast, the atoms of covalently bonded molecules (e.g., glucose, sucrose, glycerol) do not generally dissociate when placed in aqueous solution. which of the following solutions would be expected to contain the greatest number of solute particles (molecules or ions)?
a. 1 liter of 1.0 m glucose
b. 1 liter of 0.5 m NaCl
c. 1 liter of 1.0 m NaCl
d. 1 liter of 1.0 m NaCl and 1 liter of 1.0 m glucose will contain equal numbers of solute particles.
A 0.520 g sample of an unknown nonelectrolyte compound is dissolved in 4.62 g of lauric acid (Kf = 3.90 .C/m).
The freezing point depression is determine to be 4.20 C. What is the molar mass of the compound?
Answers
Using the given mass of the compound (0.520 g) and the calculated moles, we can determine the molar mass of the compound.
To find the molar mass of the compound, we can use the formula:
ΔT = Kf * m
where ΔT is the freezing point depression, Kf is the cryoscopic constant (in this case, 3.90 °C/m), and m is the molality of the solution.
First, we need to calculate the molality (m) of the solution:
m = moles of solute / mass of solvent (in kg)
The mass of the solvent (lauric acid) is given as 4.62 g. Since the unknown compound is a solute, we need to convert its mass to moles:
moles = mass / molar mass
Given that the mass of the unknown compound is 0.520 g, we can now calculate the moles of the compound.
Next, we convert the mass of the solvent to kg by dividing by 1000:
mass of solvent (lauric acid) = 4.62 g / 1000 = 0.00462 kg
Now we can calculate the molality:
m = moles of solute / mass of solvent = (moles of the compound) / (mass of solvent)
Finally, we can use the freezing point depression formula to find the molar mass of the compound:
ΔT = Kf * m
Substituting the given values:
4.20 °C = 3.90 °C/m * m
Now solve for m:
m = (4.20 °C) / (3.90 °C/m)
Once we have the molality, we can calculate the moles of the compound:
moles = molality * mass of solvent (in kg)
Finally, we calculate the molar mass:
molar mass = mass of solute / moles of solute
Learn more about molar mass here :-
https://brainly.com/question/31545539
#SPJ11
anyone? got an answer
