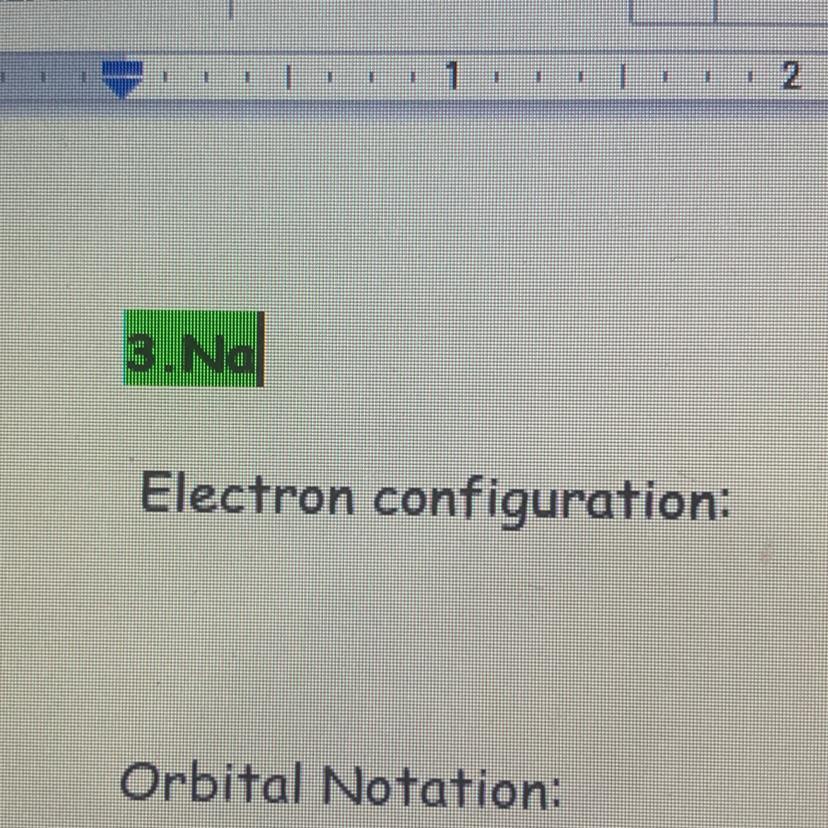
Answers
1s22s22p63s1. That's the ans
Related Questions
what happens to ammonia when reacts with heated sodium?
Answers
Answer:
When dry ammonia is passed over heated sodium in absence of air, the product formed is sodium amide. - When dry ammonia is passed over heated sodium in absence of air, sodium amide is produced. - Sodium amide aka sodamide is mainly used as a strong base in organic chemistry.
Explanation:
pls help
2.0 mol of Ca(OH)2 are mixed with 2.0 mol of HCl according to the following equation:
Ca(OH)2+2HCl=CaCl2+2H2O
a. Which chemical is in excess and which is limiting reactant?
b. What is the excess in grams?
c.Theoretically,how many moles of H20 will be produced?
Answers
Answer:
Explanation:
Limiting is HCl and excess is Ca(OH)2
excess is 296 grams Ca(OH)2
2 moles H2O will be formed
A 0.470 g sample of a metal, M, reacts completely with sulfuric acid according to M(s)+H2SO4(aq)⟶MSO4(aq)+H2(g) A volume of 217 mL of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. Atmospheric pressure is 756.0 Torr, and the temperature is 25 °C. The vapor pressure of water at 25 °C is 23.8 Torr. Calculate the molar mass of the metal.
Answers
The molar mass when rected with sulphuric acid to liberate 249 mL of hydrogen gas is 48 g/mol
The volume (V) = 249 mL = 249 / 1000 = 0.249 L
Pressure (P) = 1.0079 – 0.03167 = 0.97623 bar
The temperature (T) = 25.0 °C = 25 + 273 = 298 K
Gas constant (R) = 8.314×10¯² bar.L/Kmol
Number of mole (n) =?
n = PV / RT
n = (0.97623 × 0.249) / (8.314×10¯² × 298)
n = 0.0098 mole
How to determine the mole of the metal
Balanced equation
M(s) + H₂SO₄(aq) —> MSO₄(aq) + H₂(g)
From the balanced equation above,
1 mole of M reacts to produce 1 mole of H₂.
Therefore,
Mole of metal = 0.0098 mole
Mass of metal = 0.539 g
Molar mass is given as mass upon mole
Molar mass of metal = 0.470 / 0.0098
Molar mass of metal = 48 g/mol
Learn more about stoichiometry at:
brainly.com/question/14735801
#SPJ9
why electron affinity of nitrogen is endothermic?
Answers
Answer:
N− has the outer electron structure of 2s22p4. This means we have coulombic repulsion in one of the p orbitals which means adding a single electron will be endothermic. This accounts for the decrease in ionization energy between N and O.
Explanation:
Please mark as brainliest
Identify the substance that has formula mass of 133.5amu.
(a) MgCI
b)SCI
c)BCI
D) AICI
Answers
The calculated formula masses to 133.5 amu, we find that the substance with a formula mass closest to 133.5 amu is (d) AlCl3. Therefore, the answer is option D.
To identify the substance with a formula mass of 133.5 amu, we need to calculate the formula mass of each given substance and compare it to 133.5 amu.
(a) MgCl2:
The formula mass of MgCl2 can be calculated by adding the atomic masses of magnesium (Mg) and chlorine (Cl).
Mg: atomic mass = 24.31 amu
Cl: atomic mass = 35.45 amu
Formula mass of MgCl2 = (24.31 amu) + 2(35.45 amu) = 95.21 amu
(b) SCl:
The formula mass of SCl can be calculated by adding the atomic masses of sulfur (S) and chlorine (Cl).
S: atomic mass = 32.07 amu
Cl: atomic mass = 35.45 amu
Formula mass of SCl = 32.07 amu + 35.45 amu = 67.52 amu
(c) BCl:
The formula mass of BCl can be calculated by adding the atomic mass of boron (B) and chlorine (Cl).
B: atomic mass = 10.81 amu
Cl: atomic mass = 35.45 amu
Formula mass of BCl = 10.81 amu + 35.45 amu = 46.26 amu
(d) AlCl3:
The formula mass of AlCl3 can be calculated by adding the atomic mass of aluminum (Al) and 3 times the atomic mass of chlorine (Cl).
Al: atomic mass = 26.98 amu
Cl: atomic mass = 35.45 amu
Formula mass of AlCl3 = 26.98 amu + 3(35.45 amu) = 133.78 amu. Option D
For more such questions on masses visit:
https://brainly.com/question/24191825
#SPJ8
Which bone is located between the incus and the inner ear?
cochlea
stapes
incus
malleus
Answers
Answer: The answer is incus
The enthalpy of vaporization for methanol is 35.2 kJ/mol. Methanol has a vapor pressure of 1 atm at 64.7 oC. Using the Clausius-Clapeyron equation, what is the vapor pressure for methanol at 55.5 oC? Give your answer in atmospheres, to the third decimal point.
Answers
Answer: 55.5 oC is 0.014 atm (3rd decimal point)
Explanation:
The Clausius-Clapeyron equation is given as:
ln(P2/P1) = -(ΔH_vap/R) * (1/T2 - 1/T1)
where:
P1 = vapor pressure at temperature T1
P2 = vapor pressure at temperature T2
ΔH_vap = enthalpy of vaporization
R = gas constant = 8.314 J/(mol*K)
Converting the enthalpy of vaporization to J/mol:
ΔH_vap = 35.2 kJ/mol = 35,200 J/mol
Converting temperatures to Kelvin:
T1 = 64.7 + 273.15 = 337.85 K
T2 = 55.5 + 273.15 = 328.65 K
Substituting the values into the equation and solving for P2:
ln(P2/1 atm) = -(35,200 J/mol / 8.314 J/(mol*K)) * (1/328.65 K - 1/337.85 K)
ln(P2/1 atm) = -4.231
P2/1 atm = e^(-4.231)
P2 = 0.014 atm
Therefore, the vapor pressure for methanol at 55.5 oC is 0.014 atm, to the third decimal point.
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
How much did asbestos exposure decrease during the year 1982 and 1983

Answers
The asbestos exposure during the years 1982 and 1983 was 2.5 fibers per cubic centimeter and 0.8 fibers per cubic centimeter respectively. So asbestos exposure decreased by 1.7 fibers per cubic centimeter during the year 1983.
Breathlessness Persistent, dry cough Chest pain or tightness Lack of a dry, crackling sound in the lungs when you breathe in Wider and rounder fingers and toes are some of the symptoms of asbestos exposure.
The largest group of people exposed to asbestos is those working in the construction industry. Historically, asbestos was also used by pipe fitters and shipyard workers. In addition, asbestos was used by military personnel, auto mechanics, and many other occupations.
To learn more about asbestos, refer to the link:
https://brainly.com/question/8853025
#SPJ1
Consider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g) Calculate the volume N2O5 that must decompose completely to produce 9.64 L nitrogen dioxide.
Answers
The volume of \(N_2O_5\) needed to produce 9.64 L of \(NO_2\) is 4.97 L, calculated using stoichiometry and the ideal gas equation.
The given chemical equation is \(2N_2O_5(g) \rightarrow 4NO_2(g) + O_2(g)\) .The volume of \(N_2O_5\) that decomposes completely to form 9.64 L of \(NO_2\) is to be calculated. For this, we can use the concept of stoichiometry. Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a balanced chemical equation.To calculate the volume of \(N_2O_5\) that is needed to produce 9.64 L of \(NO_2\), we will first determine the number of moles of NO2 produced in the reaction. For this, we can use the ideal gas equation, PV = nRT. Here, we have the volume of NO2 and we can assume the pressure and temperature to be constant. Thus, we have PV = nRT, where P = pressure, V = volume, n = number of moles, R = ideal gas constant, and T = temperature. Substituting the given values in the ideal gas equation, we get,n = PV/RT = (1 atm × 9.64 L)/(0.0821 L atm K-1 mol-1 × 300 K) = 0.404 molFrom the chemical equation, we see that 2 moles of \(N_2O_5\) give 4 moles of \(NO_2\). Thus, 0.404 mol of \(NO_2\) must have been produced from (0.404/2) = 0.202 mol of \(N_2O_5\). Using the ideal gas equation, we can also find the volume of 0.202 mol of \(N_2O_5\) at the given conditions. Thus, V = nRT/P = (0.202 mol × 0.0821 L atm K-1 mol-1 × 300 K)/1 atm = 4.97 L. Thus, the volume of \(N_2O_5\) that must decompose completely to produce 9.64 L nitrogen dioxide is 4.97 L.For more questions on stoichiometry
https://brainly.com/question/14935523
#SPJ8
water vapour is the solid form of water.
True
False
Answer and I will give you brainiliest
Answers
Answer:
False
Explanation:
Water vapour exists in gas phase!
Take a balloon that we filled up with our breath. Tell me what would happen if we put it outside and let it sit in the sun for a bit. Support your answer using the KMT.Keep in mind we are going to be under the impression that no air can escape from the balloon.
Answers
The balloon will be filled by a gas, and as the gas is not escaping we can assume that it is at a constant pressure.
The KMT (Kinetic Molecular Theory) explains the macroscopic properties of gases. This theory states that gases are formed by particles in constant motion, and that their kinetic energy is directly proportional to the temperature of the gas.
If we leave the balloon filled with gas in the sun we assume that the temperature of the gas inside will start to rise. When the temperature starst to rise, and the pressure is constant, particles will gain kinetic energy. This will result in particles moving faster and coliding with each other and with the balloon's walls more often. As a consecuence the particles will tend to stay farther from one another and the volume of the balloon will increase.
This is an example of Charles's Law that states that at constant pressure the volume of a gas is directly proportional to its temperature. In other words, when the temperature of the gas increases the volume increases as well.
To summarize, if we fill a balloon and we leave it in the sun its volume will increase.
What is the liquid substance use in the laboratory for dissolving dry mortar on floor flies
Answers
The liquid substance used in the laboratory for dissolving dry mortar on floor flies is hydrochloric acid.
What is hydrochloric acid?Hydrochloric acid is a strong acid that can dissolve many materials, including dry mortar.
Hydrochloric acid also known as muriatic acid or sulfuric acid, are commonly used to dissolve hardened mortar or concrete residues.
To use hydrochloric acid to dissolve dry mortar, you will need to mix the acid with water in a ratio of 1 part acd to 10 parts water.
You should then apply the mixture to the dry mortar using a brush or spray botle.
Find more exercises on Hydrochloric acid;
https://brainly.com/question/24784580
#SPJ1
A particular medication dosage is 45.0mg/kg of body weight. what is the mass in mg of the medication a child weighing 33.5lb
Answers
Answer:
Dosage of drugs is dependent on the body weight of a person. The route of administration also plays crucial role in determining the dosage of the drugs. The calculation of dosage of drugs based on the bodyweight is calculated by the multiplication of body weight and dosage.
Explanation:
The medication dosage is given as 45mg/kg. To calculate the mass of dosage a child will require of body weight 33.5lb will be:
\(\text{Dose} = \text{Body weight} \times \text{Dosage}\)
Therefore, converting the pounds to kg, it will be:
\(33.5\;\text{lb} = 15.19\;\text{ kg} \;(1\;\text{lb} = 0.4\;\text{ kg})\)
Thus, dose of medication required will be:
\(\text{Dose} = 15.19 \: \times \: 45\)
\(\text { Dose} = 683.8\: \text { mg}\)
how many neutrons does an atom of Vanadium contain
Answers
Answer: 28
Explanation:
You want to decaffeinate your coffee by extracting the caffeine outwith dichloromethane. It's too late to extract the caffeinefrom the coffee beans because you've already brewed yourself a 200mL cup of coffee. Your particular brand of coffee contains100 mg of caffeine in that 200mL cup. The partitioncoefficient of caffeine in dichloromethane/water is 9.0.
How much caffeine would still be in your 200 mL if you did:
A. One extraction using 200 mL o fdichloro methane
B. Two extractions using 100 mL of dichloro methane each.
Answers
Solution :
The partition coefficient
\($k_d= \frac{\text{(mass of caffeine in }CH_2Cl_2 / \text{volume of }CH_2Cl_2)}{\text{(mass of caffeine in water/ volume of water)}}$\)
= 9.0
A). 1 x 200 mL extraction
Let m be the mass of caffeine in water
Mass of caffeine in \($CH_2Cl_2$\) = 100 - m
∴ \($\frac{(100-m)/200}{m/200}=9$\)
\($\frac{100-m}{m}=9$\)
\($10 \ m = 100$\)
\($m=\frac{100}{10}$\)
= 10
Therefore, the mass remaining in the coffee is m = 10 mg
B). 2 x 100 mL extraction
First extraction :
Let \($m_1$\) be the mass of the caffeine in water.
Mass of caffeine in \($CH_2Cl_2$\) = 100 - m
∴ \($\frac{(100-m_1)/100}{m_1/200}=9$\)
\($\frac{100-m_1}{m_1}=9$\)
\($5.5 \ m_1 = 100$\)
\($m_1=\frac{100}{5.5}$\)
= 18.18
Mass remaining in the coffee after the 1st extraction \($m_1$\) = 18.18 mg
Second extraction:
Let \($m_2$\) be the mass of the caffeine in water.
Mass of caffeine in \($CH_2Cl_2$\) = 18.18 - \($m_2$\)
∴ \($\frac{(18.18-m_2)/100}{m_2/200}=9$\)
\($\frac{18.18-m_2}{m_2}=9$\)
\($5.5 \ m_2 = 18.18$\)
\($m_1=\frac{18.18}{5.5}$\)
= 3.3
Mass remaining in the coffee after the 1st extraction \($m_2$\) = 3.3 mg
●
What is the mass of 7.50x1023 molecules of CaCl₂?
Answers
Answer:
138.22 g
Explanation:
First, determine the molecular weight of \(CaCl_2\):
M.W. Ca = 40.08 \(\frac{g}{mol}\)
M.W. Cl = 35.45 \(\frac{g}{mol}\)
So,
M.W. \(CaCl_2=(40.08\frac{g}{mol})+2(35.45\frac{g}{mol})\)
M.W. \(CaCl_2=40.08\frac{g}{mol}+70.90\frac{g}{mol}\)
M.W. \(CaCl_2=110.98\frac{g}{mol}\)
Recall that one mole of anything is equal to \(6.022x10^{23}\) of that thing (as one dozen is equal to 12).
So,
\(7.50x10^{23} moleculesCaCl_2(\frac{1mol}{6.022x10^{23}molecules})=1.25molCaCl_2\)
Next, determine the mass:
\(1.25molCaCl_2(\frac{110.98g}{1mol})=138.22gCaCl_2\)
In your own words, define the following terms.
star
Answers
Answer:
A Sun that is hot and big and sparkling
Explanation:
A Star is a Sun which is tended to be true
Answer:
A star is a miniature version of a sun, which burns out over a course of time.
All combustion reactions have oxygen as a reactant. (2 points)
Group of answer choices
True
False
Answers
Answer:
true
Explanation:
They all have a hydrocarbon plus oxygen.
The given statement that "all combustion reactions have oxygen as a reactant" is true. Combustion is a type of chemical reaction where a fuel (usually a hydrocarbon) combines with oxygen gas to produce heat, light, and new chemical compounds, such as carbon dioxide and water.
For combustion to occur, there must be a fuel source, oxygen, and a source of ignition, such as a spark or heat. Oxygen acts as a reactant because it combines with the fuel source to produce the new compounds. Without oxygen, the reaction cannot occur. It is important to note that not all reactions involving oxygen are combustion reactions. For example, rusting of iron is a reaction that involves oxygen, but it is not a combustion reaction.
In combustion reactions, the heat and light produced are often used for industrial processes, transportation, or heating. However, the reaction can also be destructive if not controlled, such as in wildfires or explosions. In conclusion, all combustion reactions have oxygen as a reactant, as it is necessary for the reaction to occur and produce the desired products.
For more such questions on combustion
https://brainly.com/question/13251946
#SPJ11
The number of moles of molecules in a 12.0-gram sample of Cl2 is equal too?
Answers
Answer:
0.3529moles
Explanation:
no of moles =mass ÷molar mass
molar mass =17+17
=34
12÷34
=0.3529moles
According to the mole concept, the number of moles of molecules in a 12.0-gram sample of Cl₂ is equal to 0.169 moles.
What is a mole?
Mole is defined as the unit of amount of substance . It is the quantity measure of amount of substance of how many elementary particles are present in a given substance.
It is defined as exactly 6.022×10²³ elementary entities. The elementary entity can be a molecule, atom ion depending on the type of substance. Amount of elementary entities in a mole is called as Avogadro's number.
It is widely used in chemistry as a suitable way for expressing amounts of reactants and products.For the practical purposes, mass of one mole of compound in grams is approximately equal to mass of one molecule of compound measured in Daltons. Molar mass has units of gram per mole . In case of molecules, where molar mass in grams present in one mole of atoms is its atomic mass.Number of moles is mass/molar mass= 12/70.90=0.169 moles.
Learn more about mole,here:
https://brainly.com/question/20486415
#SPJ6
Calculate the relative molecular mass of hydrated Copper (II) tetraoxosulphate (VI) CuSO4.5H₂O (Cu = 64 S = 32 H = 1 0 = 16).
Answers
The relative molecular mass of hydrated Copper (II) tetraoxosulphate (VI) CuSO4.5H₂O is 249.
What is molecular mass?Molecular mass is a measure of the total mass of one mole of a substance, which is defined as the mass of the substance divided by the number of molecules it contains. It is typically expressed in g/mol and is also known as molar mass. Molecular mass is determined by the types and number of atoms that compose a molecule, and is an important factor in understanding the properties of a substance.
This is calculated by adding the atomic masses of all the atoms present in the compound.
The atomic mass of copper is 64, sulphur is 32, oxygen is 16, and hydrogen is 1.
So, the relative molecular mass of hydrated Copper (II) tetraoxosulphate (VI) CuSO4.5H₂O is 64 + 32 + (16*4.5) + (1*5) = 249.
To learn more about molecular mass
https://brainly.com/question/24727597
#SPJ1
In what direction does thermal energy transfer?

Answers
Answer:
Either direction
Explanation:
If you cook food it would go from cold to hot. If you were to put a drink in the fridge it would go from hot to cold. So it is either direction.
Consider the fatty acid. A carboxylate salt is attached to a 19 carbon chain, where double bonds are present between carbons 5 and 6, between carbons 8 and 9, between carbons 11 and 12, and between 14 and 15. The carboxylate carbon is carbon 1. All double bonds are cis. Which of the designations are accurate for the fatty acid
Answers
Answer:
ω-3 fatty acid and 19:4(Δ5,8,11,14)
Explanation:
From the image attached below:
We can see the structure of the delta nomenclature showing the numbers of carbons, numbers of double bonds, and the locations of the double bonds in the structure of the carboxylate salt.
Also, we can see the structure of the fatty acid where; the location of the first double bond from the methyl end is shown.
Hence, the designation which are accurate for these structures are:
ω-3 fatty acid and 19:4(Δ5,8,11,14)

A radio station emits radiation at à wavelength of 3.10m. What is the station’s frequency in megahertz?
Answers
Answer: 96.7072445
Explanation: F = 1/T
What is the electron configuration for an atom on Period #3 and in Group #17?
1s22s22p63523p64523d
1s22s22p?
1s22s22p 3s23p
1s22s22p
p64523d5
Answers
Answer:
the answer the question is 2,8,7
What is the name of the following compound - AgNO3 *
Answers
Answer:
Silver Nitrate
Explanation:
What is the solubility of Mg(OH)₂ at a pH of 12.80? (Ksp Mg(OH)₂ is 1.6 × 10⁻¹³)
Answers
The calculations show that Mg(OH)₂ has a solubility of 3.5 × 10⁻⁵ M at a pH of 12.80.
The term pH, which stands for "potential of hydrogen ions," can be interpreted as a measurement of the molar concentration of hydrogen ions in a particular solution. Hence, the acidity, neutrality, or basicity of any chemical solution is often determined or specified using the power of hydrogen ions (pH).
Mg(OH)₂⇔Mg²⁺(aq)+2OH⁻(aq)
First of all, we would write the chemical equation for this chemical reaction that is appropriately balanced
The Ksp for the aforementioned chemical reaction is determined mathematically by:
Ksp = [Mg²⁺][OH⁻]²
Ksp = [x][2x]²
1.6 × 10⁻¹³ = 4x³
x = ∛4 × 10⁻¹⁴
x = 3.5 × 10⁻⁵ M.
The maximum amount of a chemical that will dissolve in a particular amount of solvent at a particular temperature is known as its solubility. Different compounds have very varying solubilities, which is a characteristic of a particular solute-solvent pair.
Learn more about solubility here
https://brainly.com/question/27278411
#SPJ1
A Gold ring is a what?
Element
Compound
Colloid
Alloy
Answers
Answer: Alloy
Explanation: gold is an alloy, or mixture of metals
What is the electron shielding effect? What is the trend for it? How and why does it happen?
Answers
Answer:
See explanation
Explanation:
In an atom, the inner electrons may shield the outer electrons from the attractive force of the nucleus. We, refer to this phenomenon as the shielding effect, It is defined as a decrease in the magnitude of attraction between an electron and the nucleus of an atom having more than one electron shell (energy level).
Shielding effect increases down the group due to addition of more shells but decreases across the period due to the increase in the size of the nuclear charge.
As the magnitude of shielding increases down the group, ionization of electrons becomes easier and the first ionization energies of elements decreases as we move down the group. Since shielding effect decreases across the period, the first ionization energies of elements increases across the period.
Which of the following gives the correct possible values of l for n = 4?
0, 1, 2, 3
–4, –3, –2, –1, 0, 1, 2, 3, 4
0, 1, 2, 3, 4
Answers
The possible values azimuthal quantum number l for n = 4 are 0 to (n-1), that is from 0 to 4.
What are quantum numbers?Quantum numbers are numbers describing the position and energy of electrons in different orbitals of an atom. An electron have a set of four quantum numbers.
The four quantum numbers are principal quantum number, azimuthal quantum number, magnetic quantum number and spin quantum number. No two electrons will have a equal set of these numbers.
The principal quantum number n is indicating the main energy level. Azimuthal quantum number l can have values from 0 to n-1. Hence, for n =4, l have values from 0 to 3. Thus option 1 is correct.
Find more on quantum numbers:
https://brainly.com/question/16977590
#SPJ1