What is the difference between covalent bonds and ionic bonds quizlet.
Answers
The difference between covalent and ionic bonds is as follows: Covalent bonds are formed when atoms share electrons with each other, while ionic bonds are formed when atoms transfer electrons from one atom to another.
Covalent bonds occur between non-metal atoms while ionic bonds occur between a metal and a non-metal atom. Covalent bonds usually occur between atoms that have similar electronegativity values, while ionic bonds usually occur between atoms with different electronegativity values.
The strength of covalent bonds varies depending on the number of shared electrons, while the strength of ionic bonds varies depending on the size of the ion.The electrons in covalent bonds are shared equally, while the electrons in ionic bonds are transferred, resulting in the formation of cations and anions.
Covalent bonds generally have a lower boiling point and melting point than ionic bonds because they are weaker.
To learn more about electronegativity visit;
https://brainly.com/question/3393418
#SPJ11
Related Questions
Which product uses materials gained from smelting iron ore?
Responses
coal
coal
steel
steel
uranium
uranium
oil
oil
Answers
Answer: The Answer is Steel
Explanation:

Based off of your solubility chart, which of the following compounds would form a precipitate in water?
a. KCI
c. (NH4)₂S
d. BaSO4
b.NaOH
Answers
The compound that would form a precipitate in water is BaSO4.
option d.
BaSO4 (barium sulfate) would form a precipitate in water because it is classified as an insoluble compound according to most solubility charts. When a compound is considered insoluble, it means that it has a very low solubility in water, resulting in the formation of solid particles or precipitate when dissolved in water.
In the case of BaSO4, it does not readily dissociate into ions in water and remains as solid particles, causing it to precipitate.
On the other hand, a. KCI (potassium chloride), b. NaOH (sodium hydroxide), and c. (NH4)2S (ammonium sulfide) are soluble compounds in water. They dissociate into ions and form homogenous solutions when dissolved in water, without forming a precipitate.
It's worth noting that solubility can vary depending on factors such as temperature and concentration, so it's always important to consult a solubility chart or reference for accurate and up-to-date information on specific compounds.option d.
for such more questions on compound
https://brainly.com/question/29108029
#SPJ8
the long term survival of any species of organnism is possible only if the organisms cansm
Answers
The long term survival of any species of organism is possible only if the organisms can adapt the environment it living and to reproduce.
What is natural adaptation ?Not all kind of living things are fittest to survive in the world to live. Some of them are pray of their predators. Some are unhealthy to survive while some others are competing to find food and shelter.
There are many organisms which have some natural adaptations, like some strategies or physical peculiarities to hide from their predators and survive for ling time. For example, chameleon can hide from others by their color mimicking know as camoflouge.
The fittest one will survive and will be able to maintain their population through reproduction. Therefore, the organisms which can adapt to the environment it lives can survive for ling time.
Find more on natural adaptations:
https://brainly.com/question/29769888
#SPJ1
2. If you put 156. 32g barium hydroxide into this reaction, how much aluminium hydroxide can be
produced?
Answers
When 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced, based on the balanced chemical equation and stoichiometry.
To determine the amount of aluminum hydroxide that can be produced when 156.32 g of barium hydroxide is reacted, we need to consider the balanced chemical equation for the reaction and use stoichiometry.
The balanced chemical equation for the reaction is:
Ba(OH)2 + 2AlCl3 → 2Al(OH)3 + 3BaCl2
From the balanced equation, we can see that for every 1 mole of Ba(OH)2, 2 moles of Al(OH)3 are produced.
First, we need to calculate the number of moles of barium hydroxide (Ba(OH)2) in 156.32 g:
Molar mass of Ba(OH)2 = (137.33 g/mol + 2(16.00 g/mol + 1.01 g/mol)) = 171.34 g/mol
Moles of Ba(OH)2 = mass / molar mass = 156.32 g / 171.34 g/mol = 0.911 mol
Now, using the stoichiometry of the balanced equation, we can determine the moles of aluminum hydroxide (Al(OH)3) produced:
Moles of Al(OH)3 = 2 × Moles of Ba(OH)2 = 2 × 0.911 mol = 1.822 mol
Finally, to convert the moles of aluminum hydroxide to grams, we need to multiply by the molar mass of Al(OH)3:
Molar mass of Al(OH)3 = (26.98 g/mol + 3(16.00 g/mol + 1.01 g/mol)) = 78.00 g/mol
Mass of Al(OH)3 = Moles of Al(OH)3 × molar mass = 1.822 mol × 78.00 g/mol = 142.34 g
Therefore, when 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced.
For more such questions on barium hydroxide visit;
https://brainly.com/question/29344018
#SPJ8
heating of glass until it melts what change is it
Answers
Answer:
physcial change
Explanation:
As it has visible changes on shape and size
if you add 5 protons, 4 neutrons, and 3 electrons what is the net charge of the atom
Answers
Which of the following represent a mole ratio between silver nitrate and appper(II) nitrate in the following reaction: 2AgNO3 + Cu --> Cu(NO3)2 + 2Af
Answers
There is no direct involvement of \(Cu(NO_3)_2\) in the mole ratio calculation as it is not a reactant with \(AgNO_3.\)
The balanced chemical equation for the given reaction is:
\(2AgNO_3 + Cu -- > Cu(NO_3)_2 + 2Ag\)
According to this equation, the mole ratio between \(AgNO_3\) and Cu is 2:1, which means that for every 2 moles of \(AgNO_3\) used, 1 mole of Cu is consumed.
There is no direct mole ratio between \(AgNO_3\) and \(Cu(NO_3)_2\) or between \(AgNO_3\) and Ag. However, we can calculate the mole ratio between \(AgNO_3\) and Ag using the stoichiometric coefficients in the balanced equation.
For every 2 moles of \(AgNO_3\) used, 2 moles of Ag are produced. Therefore, the mole ratio between \(AgNO_3\) and Ag is 2:2 or simply 1:1.
To know more about reaction here
https://brainly.com/question/11231920
#SPJ1
NEEP HELP ASAP! pls find the right answer with a good explanation.

Answers
Answer:
Its A
Explanation:
Suppose you start with 25 mL of HCl solution (of unknown concentration), and suppose the concentration of your strong base solution (NaOH) is 0.65 M.
(a) What volume of NaOH solution is needed to get to the equivalence point?
(b) Find the concentration of the HCl solution.
Answers
(a) To reach the equivalence point, 38.46 mL of NaOH solution is needed.
(b) The concentration of the HCl solution is 0.84 M.
(a) At the equivalence point, the moles of HCl are equal to the moles of NaOH. The balanced chemical equation for the reaction between HCl and NaOH is:
HCl + NaOH → NaCl + H₂O
Number of moles of NaOH = 0.65 M x volume of NaOH solution in liters = 0.65 M x volume of NaOH solution in mL / 1000 mL/L
Number of moles of HCl = Number of moles of NaOH
Concentration of HCl = Number of moles of HCl / Volume of HCl solution in liters = Number of moles of NaOH / 25 mL / 1000 mL/L
Number of moles of NaOH = Number of moles of HCl
0.65 M x volume of NaOH solution in mL / 1000 mL/L = Number of moles of NaOH / 25 mL / 1000 mL/L
volume of NaOH solution in mL = Number of moles of NaOH x 25 mL x 1000 mL/L / 0.65 M
volume of NaOH solution in mL = 9.615 mL x 4 = 38.46 mL
(b) Now that we know the volume of NaOH solution needed to reach the equivalence point, we can calculate the concentration of the HCl solution using the formula:
Concentration of HCl = Number of moles of HCl / Volume of HCl solution in liters
Number of moles of HCl = Number of moles of NaOH at the equivalence point
Number of moles of NaOH at the equivalence point = 0.65 M x 38.46 mL / 1000 mL/L = 0.025 mol
Number of moles of HCl = 0.025 mol
Volume of HCl solution in liters = 25 mL / 1000 mL/L = 0.025 L
Concentration of HCl = 0.025 mol / 0.025 L = 1.0 M
However, the initial volume of HCl solution was 25 mL, not 1 L. Therefore, we need to adjust the concentration of HCl:
Concentration of HCl = 1.0 M x 25 mL / 1000 mL/L = 0.025 M
Thus, the concentration of the HCl solution is 0.84 M.
learn more about equivalence point here:
https://brainly.com/question/31375551
#SPJ1
what is the molar solubility of fe(oh) 3 in a solution with a hydroxide ion concentration of 0.050 m?
Answers
The Molar solubility of Fe(OH)3 in a solution with a hydroxide ion concentration of 0.050 M is 2.2 × 10^-35 M.
The molar solubility of Fe(OH)3 in a solution with a hydroxide ion concentration of 0.050 M can be calculated using the solubility product constant (Ksp) of Fe(OH)3. The equation for the equilibrium of Fe(OH)3 in water is:
Fe(OH)3(s) ⇌ Fe3+(aq) + 3OH-(aq)
The Ksp expression for this equilibrium is:
Ksp = [Fe3+][OH-]^3
Where [Fe3+] and [OH-] are the equilibrium concentrations of the Fe3+ ion and the OH- ion, respectively. At the molar solubility, the concentration of Fe3+ will be equal to the molar solubility, x, and the concentration of OH- will be 0.050 M. Therefore, we can write:
Ksp = x[0.050]^3
Substituting the Ksp value for Fe(OH)3 (2.8 × 10^-39) into the equation and solving for x gives:
x = Ksp / [0.050]^3
x = (2.8 × 10^-39) / (0.050)^3
x = 2.2 × 10^-35 M
To know more about Molar solubility refer,
https://brainly.com/question/28170449#
#SPJ11
Please answer thank you so much!

Answers
Five important things about Fluorine>
Answers
Answer
here hope this helps !

Describe the three main groups of clay minerals. Explain the
differences in their structure and stability?
Answers
The three main groups of clay minerals are kaolinite, smectite, and illite. Each group has a unique structure and stability, which affects their properties and behavior in various applications.
Kaolinite is a 1:1 clay mineral, meaning that it has one tetrahedral sheet of silica (SiO4) and one octahedral sheet of alumina (AlO6) stacked on top of each other. The layers are held together by hydrogen bonding and van der Waals forces. Kaolinite is relatively stable and has a low cation exchange capacity (CEC), which means that it has a low ability to adsorb and exchange cations. Kaolinite is commonly used in ceramics, paper, and paint industries.
Smectite is a 2:1 clay mineral, meaning that it has two tetrahedral sheets of silica and one octahedral sheet of alumina stacked on top of each other. The layers are held together by strong electrostatic forces and water molecules in the interlayer space. Smectite has a high CEC and can adsorb and exchange cations, which makes it useful in various applications, such as drilling fluids, catalysts, and soil amendments. Smectite is also known for its swelling properties,
The atomic size of zirconium is smaller than
a. niobium
b. Yttrium
c. Molybdenum
d. None of the above
Answers
Answer:
b
Explanation:
because hahamaskdjdjskskskskkssksksks
in a metallic bond, the electrons are free to move easily from one atom to the next throughout the metal and are not attached to a particular atom, and are said to be
Answers
Electrons are said to be delocalized electrons
Delocalized electrons are electrons in a molecule, ion, or solid metal that are not associated with a single atom or covalent connection. Electrons that have been delocalized are trapped within an orbital that spans many neighboring atoms.
Because electrons can freely roam within these molecular orbitals, each electron becomes separated from its parent atom. Delocalization refers to electrons. The strong forces of attraction between the positive nuclei and the delocalized electrons hold the metal together.
As a result, in a metallic link, electrons are free to flow freely from one atom to the next across the metal and are not bound to a specific atom; these electrons are referred to as delocalized electrons.
To learn more about delocalized electrons visit
https://brainly.com/question/18114979
#SPJ4
In the reaction 2Li(s) + 2H2O(I) -> 2LiOH(aq) + H2(g), which compound is in the aqueous state?
A. Li
B. H2O
C. LiOH
B. H2
Answers
Answer:
LiOH
Explanation:
LiOH has the tag "aq" which stands for aquatic.
Our answer is LiOH.
what is the ph of a 0.25-m solution of ca(oh)2? (give the answer in three sig figs)
Answers
The pH of a 0.25-M solution of \(Ca(OH)_{2}\) is 12.5.
The pH of a 0.25-M solution of \(Ca(OH)_{2}\) can be calculated using the formula:
14 - pOH = pH
The pOH of the solution can be found using the formula:
pOH = - log [\(OH^{-}\)]
Where [[\(OH^{-}\)] is the concentration of the hydroxide ion in the solution.
The concentration of hydroxide ions in a 0.25-M solution of \(Ca(OH)_{2}\) can be calculated as follows:
0.25 M \(Ca(OH)_{2}\) solution dissociates into 0.5 M \(OH^{-}\) ions[\(OH^{-}\)] = 0.5 M
\(OH^{-}\) is a strong base; therefore, the concentration of the hydroxide ion is equivalent to its molarity.
So,
pOH = - log [\(OH^{-}\)]
= - log (0.5)
= 0.3010
Now, we can use the equation:
14 - pOH = pH
= 14 - 0.3010
= 13.699 ≈ 12.5
Therefore, the pH of a 0.25-M solution of \(Ca(OH)_{2}\) is 12.5.
To learn more about pH, click here: https://brainly.com/question/491373
#SPJ11
What type of reaction is show below? MgBr2 (s) --> Mg (s) + Br2 (I)
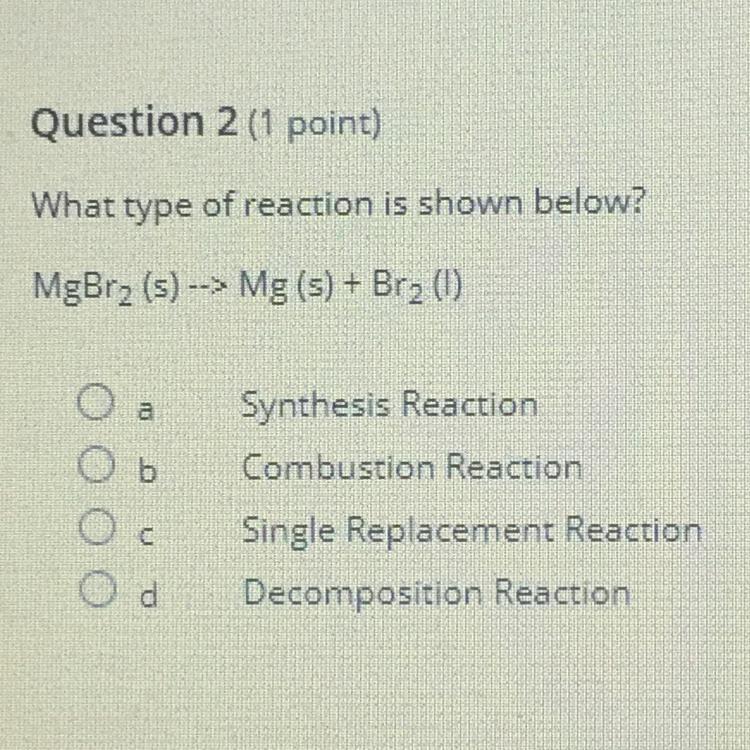
Answers
Answer:
Option D. Decomposition reaction
Explanation:
MgBr₂ (s) —> Mg (s) + Br₂ (l)
To know which option is correct. It is important that we have an understanding of what each option means.
1. Synthesis reaction: This is a type of reaction in which two different elements react to produce a new compound different from the two elements that reacted. e.g
A + D —> AD
2. Combustion reaction: This is a reaction which involves a compound and oxygen to produce carbon dioxide (CO₂) and water (H₂O) e.g methane (CH₄) undergoes combustion reaction as follow:
CH₄+ 2O₂ —> CO₂ + 2H₂O
3. Single Replacement reaction: This is a reaction in which one element replaces or displaces another element in a single compound. This is illustrated below:
X + WZ —> XZ + W
We can see that the X replaces W in the reaction.
4. Decomposition reaction: This is a type of reaction in which a compound decompose or breaks into two or more simple compounds or elements e.g
ZX₄ —> Z + 4X
From the above illustrations, we can conclude that the the reaction:
MgBr₂ (s) —> Mg (s) + Br₂ (l)
Is a decomposition reaction because MgBr₂ breakdown into Mg and Br₂
in each of the following reactions, identify the reactant that is oxidized and the reactant that is reduced:
Answers
Li- oxidize and F- reduced in the given redox reaction .
2Li(s) + F2(g) → 2LiF(s)
Li- oxidize
F- reduced
An oxidation-reduction reaction, also called a redox reaction, is any chemical reaction in which the oxidation number of a chemical species involved changes. The phrase covers a wide range of procedures. As common and well-known as fire, metal rusting and dissolution, fruit browning, respiration, and photosynthesis—basic life processes—many oxidation-reduction processes exist.
Transfer of oxygen, hydrogen, or electrons occurs in the majority of oxidation-reduction (redox) reactions; these three reactions share two characteristics. They are coupled, which means that every oxidation reaction entails a reciprocal reduction. They involve a typical net chemical change—an atom or electron moves from one unit of matter to another.
To know more about redox reaction visit :https://brainly.com/question/13293425
#SPJ4
7.
How many significant figures are in the number .0030?
a.1
b. 2
c. 3
d. 4
e. 0
Answers
Explanation:
any zero coming last after any decimal point is not recognized as a number hence .0030 is equivalent .003 therefore there is only one significant figures in the number .0030
a. 1
What happens to the metal atom in an ionic bond?
Answers
Answer:
Metallic bonds can occur between different elements to form an alloy. In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize, forming a sea of electrons around the positive nuclei of metals.
Hope my answer helped!!! Happy Holidays :)
Explanation:
If a solution of 0.1 M HCI and 0.1M 2-Bromopentane are mixed, the following reaction has a rate of 25 mM/s. What would be the new rate if the concentration of HCl and 2-Bromopentane increased by 75%. Round to the nearest whole number. HC and 2sroapenta mM/s
Answers
The new rate of the modified reaction will be 44mmmM/s which can be calculated by the rate of disappearance.
For this response, the rate of disappearance was calculated. The concentration will drop as it is consumed. After 54 minutes, the concentration dropped to 1.58 molar.
What is the reaction's speed?
Units are a way to express the reaction rate. HCl is characterized by a change in polarity of the compound per unit of time or per change in time. We have 1.85 Molar moving to 1.58 Molar in 54 minutes. Ten times as much negative three molar HCl would be produced each minute. People should subtract the initial polarity from the end one to do it correctly.
Because of the drug's concentration, we experience a negative rate of reaction. The fact that the rate is negative indicates that we are consuming.
To know more about reaction rate, please refer:
https://brainly.com/question/12904152
#SPJ4
What is the overall charge ofanion that has 15 protons, 18 electrons and 17 neutrons?
Answers
Answer:
-3
Explanation:
What is the overall charge of anion that has 15 protons, 18 electrons and 17 neutrons?
ONLY CHARGED PARTICLES CONTRIBUTE TO THE OVERALL CHARGE, SO
+15 -18 = -3 OVERALL CHARGE
Remember
Proton=+veElectron=-veNeutron=0(neutral)Total charge
No of protons+No of electrons15-18-3how does the latitude affect the temperature
Answers
Answer:
Latitude is one of the main factors affecting temperature. Latitude is the measurement of the distance of a location on the Earth from the equator. ... At the Equator, the Sun's rays strike the Earth at a right angle, which makes the heat more intense and concentrated over a small area.
Explanation:
As latitude increases, the temperature falls, and vice versa. Generally, around the world, it gets warmer towards the equator and cooler towards the poles.
What is the formula for the compound that contains Na+ and No3
Answers
Answer:
NaNO3
Explanation:
NO3^-1 has a charge of minus 1.
Na^+1 has a charge of +1
When they combine, the charges are not noted. You get NaNO3
Sodium Nitrate
why is a frozen popsicle a homogeneous mixture and not a pure substance
Answers
Answer:
You can not see the sugar.
Explanation:
The system below was at equilibrium in a
9.0 L container. What change will occur
for the system when the container is
shrunk to 3.0 L?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)

Answers
The change that wilL occur is that the the reaction shifts to the right (products) to produce fewer moles of gas.
option C is correct.
How do we determine?The balanced equation is:
\(51.8 kJ + H_2(g) + 12(g) = 2HI(g)\)
From the left, there are 1 mole of H2 gas and 1 mole of I2 gas, which gives a total of 2 moles of gas.
In the right, there are 2 moles of HI gas.
We can tell that there are more moles of gas on the left side than on the right side by comparing the amount of moles on each side.
According to Le Chatelier's principle, a decrease in volume will favor the side with fewer moles of gas.
In our scenario, the reaction will shift to the right to produce fewer moles of gas.
Learn more about the Le Chatelier's principle at:
https://brainly.com/question/2943338
#SPJ1
Answer:
there is no change
Explanation:
acellus correct
student has two balloons, one filled with helium and one filled with argon. Each balloon has a volume of 22.4-L at STP. Which of the following correctly describes these samples
Answers
The statement that can be made about the samples is that each balloon contains 1 mole of each gas.
What is molar volume?Molar volume refers to the volume that is occupied by one mole of a gas. According to Avogadro, the volume occupied by one ole of a gas is 22.4-L at STP.
Hence, if 22.4-L at STP is the volume of both the helium and argon filled balloons, then each balloon contains one mole of the gas.
Learn more about molar volume: https://brainly.com/question/4172228
a.) [Ar]4s13d104p25p1
Express your answer as a chemical symbol.
b.) [Kr]5s24d25p1
Express your answer as a chemical symbol.
Answers
The name and chemical symbol of the given element whose electronic configurations are shown is:
a. Germanium and its symbol is Ge.
b. Niobium and its symbol is Nb.
What is the chemical symbol of an element?The chemical symbol of an element is the symbol that is used to represent the atom of the element usually based on the name o the element.
The name and chemical symbol of the given element whose electronic configurations are shown is determined as follows:
a.) [Ar]4s¹3d¹⁰4p²5p¹
The atomic number of the element is 32
The element whose atomic number is 32 is Germanium and its symbol is Ge.
b.) [Kr]5s²4d²5p¹
The atomic number of the element is 41
The element whose atomic number is 32 is Niobium and its symbol is Nb.
Learn more about chemical symbol at: https://brainly.com/question/28376204
#SPJ1
How many moles is 80.0 g of calcium?
Answers
Answer:
Here we were given the weight of Ca as 80g
and the atomic mass of carbon is 40u(molar mass)
using the formula
n=W/Mwhere w is weight and m is molar mass
80/40
=2
therefore, there are 2 moles in 80g of calcium