Answers
Answer:
The answer is 6 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\ \)
From the question we have
\(density = \frac{18}{3} \\ \)
We have the final answer as
6 g/mLHope this helps you
Related Questions
Convert 2.40 x 10 23 molecules of an anonymous substance with a molar mass of 18.02 g/mol to its mass in grams.
Answers
The mass of 2.40 × 10²³ molecules of anonymous substance with molar mass of 18.02g/mol is 7.18grams.
How to calculate mass?The mass of a substance can be calculated by multiplying the number of moles in the substance by its molar mass as follows:
mass = no of moles × molar mass
First, we convert the number of molecules in the anonymous substance to moles as follows:
2.40 × 10²³ ÷ 6.02 × 10²³ = 0.39moles
mass of anonymous substance = 0.39 moles × 18.02g/mol = 7.18grams
Learn more about mass at: https://brainly.com/question/13320535
#SPJ1
Heredity Lab Report Instructions:
In the Heredity lab, you investigated how hamsters inherit traits from their parents. Record your observations in the lab report below. You will submit your completed report.
Name and Title: Include your name, instructor's name, date, and name of lab.
Objective(s): In your own words, what was the purpose of this lab?
Hypothesis: In this section, please include the if/then statements you developed during your lab activity.
These statements reflect your predicted outcomes for the experiment.
Test One: If I breed a short fur, FF female with a short fur, Ff male, then I will expect to see (all short fur; some short and some long fur; all long fur) offspring.
Test Two: If I breed a short fur, Ff female with a short fur, Ff male, then I will expect to see (all short fur; some short and some long fur; all long fur) offspring.
Test Three: If I breed a long fur, ff female with a long fur, ff male, then I will expect to see (all short fur; some short and some long fur; all long fur) offspring.
Procedure: The procedures are listed in your virtual lab. You do not need to repeat them here.
Please be sure to identify the test variable (independent variable) and the outcome variable (dependent variable) for this investigation. Remember, the test variable is what is changing in this investigation.
The outcome variable is what you are measuring in this investigation.
Test variable (independent variable): Outcome variable (dependent variable): Data: Record the data from each trial in the data chart below. Be sure to fill in the chart completely. Test One Parent 1: FF Parent 2: Ff Phenotype ratio: ________ : ________ short fur : long fur Test Two Parent 1: Ff Parent 2: Ff Phenotype ratio: ________ : ________ short fur : long fur Test Three Parent 1: ff Parent 2: ff Phenotype ratio: ________ : ________ short fur : long fur Conclusion: Your conclusion will include a summary of the lab results and an interpretation of
Answers
For Test One, phenotype ratio is Short fur : Long fur = 2 : 0; For Test Two, the phenotype ratio is Short fur : Long fur = 3 : 1; For Test Three, the phenotype ratios will be Short fur : Long fur = 0 : 2
What are the phenotype ratios from the test crosses?For Test One:
Parent 1: FF (homozygous dominant for short fur)
Parent 2: Ff (heterozygous for short fur)
The Punnett square for this cross will give the following genotype ratios:
FF : Ff = 1 : 1
And the corresponding phenotype ratios will be:
Short fur : Long fur = 2 : 0 or 100% short fur
For Test Two:
Parent 1: Ff (heterozygous for short fur)
Parent 2: Ff (heterozygous for short fur)
The Punnett square for this cross will give the following genotype ratios:
FF : Ff : ff = 1 : 2 : 1
And the corresponding phenotype ratios will be:
Short fur : Long fur = 3 : 1 or 75% short fur and 25% long fur
For Test Three:
Parent 1: ff (homozygous recessive for long fur)
Parent 2: ff (homozygous recessive for long fur)
The Punnett square for this cross will give the following genotype ratios:
ff : ff = 1 : 0
And the corresponding phenotype ratios will be:
Short fur : Long fur = 0 : 2 or 100% long fur
For this investigation, the test variable is the breed of hamster and the outcome variable is the phenotype of the hamster.
Learn more about heredity at: https://brainly.com/question/930755
#SPJ1
what is the mass of an iridium sample which absorbs 895 j of energy when its temperature increases by 12.3 C
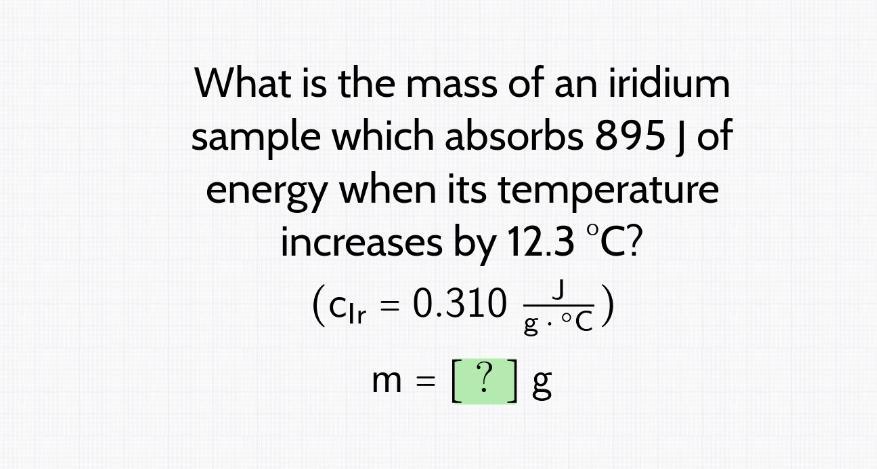
Answers
The mass of an iridium sample which absorbs 895J of energy when its temperature increases by 12.3°C is 234.72g.
How to calculate mass?The mass of a substance can be calculated using the following calorimetry equation:
Q = mc∆T
Where;
Q = quantity of heat absorbed or released (J)m = mass of substancec = specific heat capacity (J/g°C)∆T = temperature (°C)According to this question, an iridium sample absorbs 895J of energy when its temperature increases by 12.3°C. The mass can be calculated as follows:
895 = m × 0.310 × 12.3
895 = 3.813m
m = 234.72g
Therefore, 234.72g is the mass of the iridium sample.
Learn more about mass at: https://brainly.com/question/28992424
#SPJ1
How many protons, neutrons and electrons will and atom have with an atomic number of 75 and and atomic mass of 150?
Answers
Answer:
proton = 75
electron = 75
neutron = 75
Explanation:
because
proton = electron = atomic number
neutron = mass number - proton
plz follow me
Can someone please help me with EARTH SCIENCE, in New York State?! I need you to label each box with a number. it’s called “rock strata correlation practice”. Number 1 would be the oldest when u label it and it’d be at the bottom.


Answers
In the rock strata correlation practice for New York State, you'll need to label each box with a number, with number 1 representing the oldest layer, located at the bottom.
To perform the correlation, start by examining the different rock layers across different locations or outcrops in New York State. Compare the characteristics of these layers, such as their composition, fossil content, and sedimentary structures. Look for similarities and patterns between the layers to determine their correlation. By identifying key markers, such as unique fossils or distinctive sedimentary structures, you can establish relationships between the rock layers at different locations. Remember to consider the principle of superposition, which states that younger rocks are typically found above older rocks unless they have been disturbed by geological processes. Applying these principles will help you accurately correlate the rock strata in New York State.For such more question on rock strata
https://brainly.com/question/29976831
#SPJ8
Name the following alkyne:
CH3
|
CH3CH2C = CCH2CH2CHCH3
=

Answers
Answer
D. 7-methyl-3-octyne
Explanation
In naming Alkynes, the following rules stand:
Rule 1: Find the longest carbon chain that includes both carbons of the triple bond.
Rule 2: Number the longest chain starting at the end closest to the triple bond. A 1-alkyne is referred to as a terminal alkyne and alkynes at any other position are called internal alkynes.
Rule 3: After numbering the longest chain with the lowest number assigned to the alkyne, label each of the substituents at its corresponding carbon. While writing out the name of the molecule, arrange the substituents in alphabetical order. If there are more than one of the same substituents use the prefixes di, tri, and tetra for two, three, and four substituents respectively. These prefixes are not taken into account in alphabetical order.
The give alkyne has 8 longest carbon chain that includes the carbons with the triple bond. Carbon number 3 has the triple bond and a methyl substituent is bonded to carbon number 7 as shown below:
8 carbon chain with triple bond on carbon 3 -the parent name will be 3-octyne.
A methyl substituent is on carbon 7. Hence the name of the alkyne will be: 7-methyl-3-octyne.

Answer:D. 7-methyl-3-octyne
A fire women dropped a person onto the safety net.Right before the person hit the net he had a velocity of 11.2m/s and 1800 J of kinetic energy. What was the mass of the person?
Answers
Explanation:
kinetic energy = ½mv²
1800 = ½ × m × 11.2²
1800 = ½ × m × 125.44
3600 = 125.44m
m = 3600/125.44
m = 28.7 Kg
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
What is the electron geometry and hybrid orbital of sio2
Answers
Answer:
linear electron and molecular geometry with a bond angle of 180 degrees and a hybridization of Sp
Explanation:
What is the name in a position called

Answers
The names of the positions are called:
(1) (10) Atomic number
(2) (11) Chemical symbols
(3) (12) Elements
(4) (13) Atomic mass
What is an atomic structure?Atomic structure refers to the composition and arrangement of subatomic particles within an atom. An atom consists of a central nucleus, which contains positively charged protons and uncharged neutrons, surrounded by negatively charged electrons that move around the nucleus in shells or energy levels.
The number of protons in the nucleus determines the atomic number and thus the identity of the element. The arrangement of electrons around the nucleus determines the chemical and physical properties of the element.
Learn more on atomic structure here: https://brainly.com/question/21289019
#SPJ1
The questions are:
10 What is the name for the number in this position called? (the answer is not "6") →6
11 What is the name for the letter in this position called? (the answer is not "C"!) →C
12 What is the name in this position called? (the answer is not "Carbon"!) →Carbon
13
What is the name for the number in this position? (the answer is not "12.0") →12.0
Use the spaces below to type your answers to the questions above.
Convert 675000 to scientific notation
Answers
Answer:
To convert 675000 to scientific notation, we need to express it in the form a × 10^n, where a is a number between 1 and 10 (but not 10 itself), and n is an integer.
Starting with 675000, we can divide by 10 repeatedly until we get a number between 1 and 10.
675000 ÷ 10 = 67500 (one division by 10)
67500 ÷ 10 = 6750 (two divisions by 10)
6750 ÷ 10 = 675 (three divisions by 10)
Now we have a number between 1 and 10 (namely, 6.75), and we know that we divided by 10 three times, so the exponent is -3.
Therefore, we can express 675000 in scientific notation as:
6.75 × 10^5
(Note that we could also express it as 6.75 × 10^2 × 10^3, but this is not in standard scientific notation, which requires the coefficient to be between 1 and 10.)
What was earth’s surface like? Landmasses? First land plants
Answers
Answer:
During the early Paleozoic Era, the Earth's surface was very different from what it is today. The continents were arranged differently, forming one large supercontinent called Pangea. This landmass was surrounded by a single large ocean called Panthalassa. The climate was much warmer and wetter than it is today, with no ice caps at the poles.
The first land plants, known as bryophytes, appeared during the early Silurian Period, around 430 million years ago. These plants were small and simple, lacking roots and vascular tissue. They grew in damp environments, such as along the edges of lakes and streams. They were important in the development of soils and in the colonization of land by other organisms, such as insects and other arthropods.
Check
Match each power of a power expression with its simplified expression.
(4-3)-3
(40)-9
(46)-3
(-49)2
ТТІ
49
(-4)18
1
40
Answers
The simplified expression for the given expression is:40T^2 × T^98 × (4^181) / 4^67
Given expression : (4^-3)^-3 × (4^0)^-9 × (4^6)^-3 × (-4)^-49 × (2T)^2 × (T^2)^49 × (-4)^181 × 40To simplify the given expression, we use the following properties of exponents : For any real numbers a, b and n, we have ;a^-n = 1/a^n and a^n × a^m = a^(n+m)Let's simplify each term of the given expression one by one:(4^-3)^-3 = 4^(9) because when a negative exponent is raised to another negative exponent, it becomes positive. (4^-3)^-3 = 4^(-3×-3) = 4^(9)(4^0)^-9 = 4^0 = 1 because any number raised to the power of 0 is equal to 1(4^6)^-3 = 4^(-6×3) = 4^(-18) because when a negative exponent is multiplied by another negative exponent, it becomes positive.(-4)^-49 = -1/(4^49) because when a negative exponent is raised to another negative exponent, it becomes positive and also negative.(-4)^181 = (4^181) because when an odd negative power of a negative number is raised to another power, it becomes negative.40 = 40 as it is(2T)^2 = 4T^2(T^2)^49 = T^(2×49) = T^98(-49) = -49 as it is Now let's simplify the given expression:1 × 1/(4^49) × 4^(-18) × 40 × 4T^2 × T^98 × (4^181)40 and 4^-18 can be simplified and combined as follows:1/(4^49) × 4^(-18) × 40 = 40/(4^49 × 4^18) = 40/4^(49+18) = 40/4^67.
for such more questions on expression
https://brainly.com/question/31591125
#SPJ8
123.0 x 12.35 / (0.05 x 6.049) significant figures
Answers
Answer:
123.0*12.35/(0.05*6.049)
first do in small bracket 123.0*12.35/0.30245
and then divide 123.0*40.83319557
and multiply 5022.483055
Part 1(Picture 1):
Peptides isolated from rapeseed that may lower blood pressure have the following sequence of amino acids.
Part A
At physiological pH the N-terminus of an amino acid exists as the ammonium ion, and the C-terminus exists as the carboxylation ion.
Draw the structure of Arg-Ile-Tyr.
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars, including charges where needed.
Part 2(Picture 2):
Part A
What are the amino acids in the peptide?
Spell out the full names of the compounds. Enter your answers separated by a comma.
Part B
How would you name the dipeptide in the peptide?
Spell out the full name of the compound.


Answers
The structure of Arg-Ile-Tyr is given below:
What are Peptides?Peptides make up short chains of amino acids, which act as the fundamental units for building proteins. Within these chains, peptides usually consist of no more than 50 amino acids in contrast to proteins that are made up of significantly longer structures.
Found throughout various sources such as plants, animals, and bacteria, peptides play crucial roles in multiple biological processes including signaling, enzyme activity, and immune response.
Additionally, artificially synthesized peptides serve several purposes within the medicine, cosmetics, and food industries. Scientists are also exploring certain peptide's potential therapeutic advantages mainly pertaining to anti-inflammatory, antimicrobial, and anticancer activities.
Read more about peptides here:
https://brainly.com/question/21884818
#SPJ1


A compound is brittle and does not conduct electricity in the solid state. It dissolves inwater and the solution conducts electricity. What type of bonding does it likely have?a. Covalentb. Ionicc. metallic
Answers
Explanation
Ionic solids, such as sodium chloride (NaCl) are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite strong.
Many ionic crystals also have high melting points. This is due to the very strong attraction between the ions.
Although they are hard they also tend to be brittle, and they shatter rather than bend. Ionic solids do not conduct electricity; however, they do conduct when molten or dissolved because their ions are free to move. Many simple compounds formed by the reaction of a metal and a nonmetal element are ionic.
Answer: b. Ionic
What is the balanced equation for the redox reaction between zinc and hydrochloric acid (HCl) that forms zinc(II) ions and hydrogen gas? A. 2Zn + HCl + H+ Zn2Cl + H2 B. Zn + 2H+ 2Zn2+ + H2 C. 2Zn + 2H+ 2Zn2+ + H2 + 2e- D. Zn + 2H+ 2Zn2+ + H2 + 2e-
Answers
The balanced equation for the redox reaction between zinc and hydrochloric acid (HCl) that forms zinc(II) ions and hydrogen gas is as follows:
Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
What is a redox reaction?A redox reaction is a type of chemical reaction in which some of the atoms have their oxidation number changed.
According to this question, the redox reaction between zinc and hydrochloric acid (HCl) forms zinc(II) ions and hydrogen gas. The balanced equation is as follows:
Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
Learn more about redox reaction at: https://brainly.com/question/13293425
#SPJ1
16.4 A cylinder of water contains oxygen in solution. The cross- sectional area of the cylinder is 2 cm2 and the length of the cylin- der is 5 cm. At one end of the cylinder the concentration of oxygern is maintained at 0.2 mol m-3, this concentration falls linearly to 0.05 mol m-3 at the other end of the cylinder. The diffusion con- stant of oxygen in water is 8 × 10-10 m2 s-1. How many moles of oxygen pass down this cylinder every second? What mass of oxy- gen passes down the cylinder each second?
Answers
Answer:
A) oxygen moles/sec = 48 * 10 ^-14 mol/sec
B) mass of oxygen = 1536*10^-14 grams
Explanation:
cross sectional area of cylinder = 2cm^2 = 2 * 10^-4 m^2
length of cylinder = 5 cm = 0.05 m
concentration at one end = 0.2 mol m^-3
concentration falls linearly at other end = 0.05 mol m^-3
Diffusion constant of oxygen = 8 * 10^-10 m^2 s^-1
A ) Number of moles of oxygen passing every second
N = A * D * \(\frac{dc}{dt}\) ------- ( 1 )
A = area , D = diffusion constant, \(\frac{dc}{dt}\) = rate of change of diffusion
dc /dt = ( 0.2 - 0.05 ) / 0.05 = 3 mol m^-4
back to the equation
N = ( 2 * 10^-4 ) * ( 8 * 10^-10 ) * ( 3 ) = 48 * 10 ^-14 mol/sec
B) Mass of oxygen passing down the cylinder each second
This can be obtained by converting : 48 * 10^-14 moles to grams
1 mole of oxygen = 32 grams of oxygen
therefore : 48 * 10^-14 moles of oxygen = ( 32 * (48*10^-14) = 1536*10^-14 grams
a compound has a molecular formular of C12H24O6.What is the compound's empirical formula
Answers
Answer:
The empirical formula for C12 H24 O6 is C2 H4 O.
Answer:
We are given the formula of the compound:
C12H24O6
The empirical formula of a molecular formula is the lowest whole number ratio between the number of atoms of each element
The ratio of C to H to O in the given formula is :
12 : 24 : 6
we notice that all 3 of the numbers have 6 in common. Dividing all three of the numbers by 6, we get:
2 : 4 : 1
Hence, the ratio of Carbon to Hydrogen to Oxygen in the empirical formula of the given compound is 2 : 4 : 1 ,
Empirical Formula = C2H4O
An ion
A. is an ion with a negative charge
B. attracts ions with a negative charges
C. results when an alkaline-earth metal loses one of its outermost electrons
D. has more protons than electrons
Answers
Answer:
C. results when an alkaline-earth metal loses one of its outermost electrons
Explanation:
An ion can be said to result when an alkaline - earth metal loses one of its outermost electrons.
Ions are charged substances that takes part in chemical reaction.
An an atom is neutral substance that is a component of an element. An ion is charged substance. In an ion, the number protons and electrons are unbalanced. The number of protons in an atom are the positively charged particles. The number of electrons are the negatively charged particles.When there is an inequality between the number of protons and electrons within an atom, an ion forms.
The reaction
C(s)+2H2(g)⇌CH4(g)
has p=0.263 at 1000. K. Calculate the total pressure at equilibrium when 4.553 g of H2 and 22.14 g of C(s) are placed in an 8.89 L flask and heated to 1000. K.
Ptotal=______ atm
Answers
From he calculations, we can see that the total pressure at equilibrium is 21 atm.
What is equilibrium constant?The term equilibrium constant commonly describes the constant that that shows the extent of conversion of reactants to products.
We have to find the pressure of each gas as follows;
For H2
P = nRT/V = 4.553 /2 × 0.082 × 1000/8.89 L = 21 atm
Using the ICE table;
C(s) + 2H2(g) ⇌ CH4(g)
I 21 atm 0
C -x +x
E 21 - x x
0.263= x/(21 - x )^2
0.263(21 - x )^2 = x
38 - 11x - 0.263x^2 = x
0.263x^2 + 12x - 38 = 0
x=2.97 atm
At equilibrium, we have;
(21 - 2.97) + 2.97 = 21 atm
Learn more about equilibrium constant: https://brainly.com/question/17960050
Classify the following mixtures as homogeneous or heterogeneous. Drag the appropriate items to their respective bins. View Available Hint(s) Reset Help brass gravel vodka potato salad sugar STUP Homogenous mixture Heterogeneous mixture
Answers
Homogeneous mixture are Brass, Vodka and Sugar; while Heterogeneous mixture are Gravel and Potato salad.
A homogeneous mixture is a type of mixture where the composition is uniform and the same throughout. Examples of homogeneous mixtures include saltwater, air, and vinegar. These mixtures have a consistent appearance and properties, and their individual components cannot be easily separated.
A heterogeneous mixture is a mixture where the composition is not uniform and can vary in different parts of the mixture. Examples of heterogeneous mixtures include salad, fruit punch, and soil. These mixtures have varying appearance and properties, and their individual components can be easily separated.
You can learn more about Homogenous mixture & Heterogeneous mixture at
https://brainly.com/question/14441492
#SPJ4
Describe some of the types of power reactors that have been designed.
Answers
Answer:
(Magnox, AGR, PWR, BWR, CANDU and RBMK) have emerged as the designs used to produce commercial electricity around the world. A further reactor type, the so-called fast reactor, has been developed to full-scale demonstration stage.
Explanation:
Answer:
A pressurized-water, graphite-moderated reactor shielded by concrete
A graphite-moderated reactor that uses enriched uranium fuel
A reactor that uses liquid sodium as a coolant
A reactor that uses moderately enriched uranium as the fuel and ordinary water as the moderator in place of graphite
(01.01 LC)What is the body of scientific knowledge based on?
Guesses
Mysteries
Observations
Opinions
Answers
The body of scientific knowledge is based on different Observations (Option C).
What does observations mean in the scientific method?Observations in the scientific method are fundamental because it is the first step to raising scientific questions that may be explained through plausible hypotheses. Subsequently, hypotheses must be tested by experimental procedures.
In conclusion, the body of scientific knowledge is based on different Observations (Option C).
Learn more about observations in the scientific method here:
https://brainly.com/question/2505873
#SPJ1
Plzzzzzzzzzzzzz help

Answers
Answer:
A layer of soft hot rock
Explanation:
How did Dmitri Mendeleev arrange the periodic law?
a. Each set of elements was arranged in special columns based on their mass.
b. Each set of elements was arranged in special columns based on their diameter.
c. Each set of elements was arranged in special periods based on their qualities.
d. Each set of elements was arranged in alphabetical order.
Answers
Answer:
a. Each set of elements was arranged in special coloumns based on their mass.
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
Draw a structural formula for the major product of the reaction shown.
Answers
Draw a structural formula for the major product of the reaction shown:
The structural formula for the major product (2-butene) of the given reaction is as follows:$$\ce{CH3CH2CH=CH2}$$
The given reaction is an acid-catalyzed dehydration reaction.
During the reaction, the hydroxyl group (OH) and the adjacent hydrogen atoms (H) on the reactant alcohol (2-butanol) undergo dehydration (loss of water) to form an alkene (2-butene) as the major product.
The reaction is shown below:$$\ce{CH3CH2CH2CH2OH + H2SO4 ->[\Delta] CH3CH2CH=CH2 + H2O}$$To draw the structural formula for the major product of the given reaction, we need to consider the following points:
1. The reactant alcohol (2-butanol) is a four-carbon alcohol with the hydroxyl group (OH) attached to the second carbon atom (C2) of the chain.
2. The product alkene (2-butene) will be a four-carbon alkene with a double bond between the second and third carbon atoms (C2 and C3) of the chain.
The other two carbon atoms will have a single bond with the adjacent carbon atoms and a hydrogen atom each attached to them.
3. The major product will be formed via the elimination of water (dehydration) between the hydroxyl group (OH) and the adjacent hydrogen atoms (H) on the second carbon atom (C2) of the reactant alcohol (2-butanol).
4. The acid catalyst (H2SO4) does not participate in the reaction and remains unchanged. It only facilitates the formation of the alkene by providing a proton (H+) to the hydroxyl group (OH) and a medium for the elimination of water.
For more such questions on alkene
https://brainly.com/question/27704061
#SPJ8
Determine the name or formula for each polyatomic ion.
formula: PO3−4
name:
name: sulfite ion formula:
name: sulfate ion formula:

Answers
Answer:
See explanation
Explanation:
PO4{3-} is phosphate
Sulfite's formula is SO3{2-}
Sulfate is SO4{2-}
OH- is hydroxide
Note: {x±} signifies the charge of the entire molecule
The polyatomic ions in question are phosphite ion, sulfite ion, and sulfate ion.
Explanation:The formula PO3−4 represents the polyatomic ion called phosphite ion. It is composed of one phosphorus atom bonded to three oxygen atoms. The name of the sulfite ion is SO3−2, and it consists of one sulfur atom bonded to three oxygen atoms. Lastly, the sulfate ion has the formula SO4−2, and it is composed of one sulfur atom bonded to four oxygen atoms.
Learn more about Polyatomic ions here:https://brainly.com/question/35456287
#SPJ6
As the density of a substance decreases, the volume of a given mass of that
substance _________.
A. increases
B. is not affected
C. decreases
Answers
Mark me a brainliest :)