Answers
Answer:
FeP, which is iron (III) phosphide.
Explanation:
Hello,
In this case, when iron (III) ion and phosphide ion react, the following compound is formed as the charges are exchanged to each other at the subscripts:
\(Fe^{3+}+P^{3-}\rightarrow Fe_3P_3\)
Yet, since the three could be simplified to 1 at both Fe and P, we obtain:
\(Fe^{3+}+P^{3-}\rightarrow FeP\)
Which is known as iron (III) phosphide.
Best regards.
Related Questions
What volume of 12M HCI is needed to prepare 250
of 0.20M HCI?
Answers
Answer: 4.2
Explanation:
\(M_{A}V_{A}=M_{B}V_{B}\\(12)V_{A}=(250)(0.20)\\V_{A}=\frac{(250)(0.20)}{12}=\boxed{4.2}\)

What challenges did scientists across the world face that inspired them to create the SI?
Answers
Answer:
SI has seven basic units, from which others are derived: the second, ... of the metric system had grown out of the problems scientists encountered.
Explanation:
I learned this in school
Write the chemical symbols for three different atoms or atomic anions with 23 electrons.
Answers
Krypton, Chromium, and Oxygen with the following symbols Kr-13, Cr-2, and O-15 respectively have 23 electrons.
The atomic number of an atom determines the number of electrons it has. When the number of protons is equivalent to the number of electrons, the atom is electrically neutral. An anion, on the other hand, is an atom with a negative charge. It has gained an electron or two, or even more. Below are the chemical symbols for three different atoms or atomic anions with 23 electrons.Krypton:Kr has an atomic number of 36, indicating that it has 36 electrons. However, if we add 13 electrons to it, the total number of electrons becomes 49. Krypton with 13 additional electrons becomes Kr-13, with a total of 49 electrons.Chromium:Cr has an atomic number of 24, indicating that it has 24 electrons. Adding two more electrons to it, the total number of electrons becomes 26. The atomic anion with 26 electrons is Cr-2.Oxygen:Oxygen has an atomic number of 8, indicating that it has 8 electrons. However, if we add 15 electrons to it, the total number of electrons becomes 23. Oxygen with 15 additional electrons becomes O-15, with a total of 23 electrons.
for more questions on electrons
https://brainly.com/question/371590
#SPJ8
Determine the type of reaction: AgNO3 + Cu --> Cu(NO3)2 + Ag
Answers
Explanation:
Cu + 2 AgNO3 → Cu(NO3)2 + 2 Ag
Cu is oxidized
Ag+ is reduced
Cu is the reducing agent
To solve such this we must know the concept of displacement reaction. The balanced reaction of silver nitrate with copper is of displacement type of reaction.
AgNO\(_3\) + Cu \(\rightarrow\) Cu(NO\(_3\))\(_2\) + Ag
What is Balanced equation?Balanced equation is the one in which the total number of atoms of a species on reactant side is equal to the total number of atoms on product side. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, displacement reaction.
The reaction between silver nitrate and copper is example of displacement reaction. Copper displaces silver from silver nitrate. to form copper nitrate.
Therefore, the balanced equation is of displacement type of reaction.
AgNO\(_3\) + Cu \(\rightarrow\) Cu(NO\(_3\))\(_2\) + Ag
Learn more about the balanced equation, here:
https://brainly.com/question/7181548
#SPJ2
A piece of metal weighing 59.0 g was heated to 100 C and then put into 100.0 g of water (initially at 23.0 C) the metal and water were allowed to come to an equilibrium temperature, determined to be 27.5 C. Assuming no heat lost to the environment, calculate the specific heat of the metal.

Answers
We have a hot piece of metal that is put in water, and the metal and water are allowed to come to an equilibrium. We can consider that no heat is lost. So the amount of heat that the piece of metal is losing, is gained by the water. The piece of metal is heating the water. We can write that as:
Q water = - Q metal
Then the general formula for the heat of anything is:
Q = m * C * ΔT
So:
Qwater = - Qmetal
mw * Cw ΔTw = -
What's galactose's empirical formula?
Answers
Answer:
C6H12O6
Explanation:
C6H12O6
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
With 21 g of Zinc, and 7 g of CuCl2, how much ZnCl2 is made in grams?
Answers
Answer: 7.07 grams
Explanation:
To calculate the moles :
\(\text{Moles of solute}=\frac{\text{given mass}}\times{\text{Molar Mass}}\)
\(\text{Moles of} zinc=\frac{21g}{65g/mol}=0.32moles\)
\(\text{Moles of} CuCl_2=\frac{7g}{134g/mol}=0.052moles\)
\(Zn+CuCl_2\rightarrow Cu+ZnCl_2\)
According to stoichiometry :
1 mole of \(CuCl_2\) require 1 mole of \(Zn\)
Thus 0.052 moles of \(CuCl_2\) will require=\(\frac{1}{1}\times 0.052=0.052moles\) of \(Zn\)
Thus \(CuCl_2\) is the limiting reagent as it limits the formation of product and \(Zn\) is the excess reagent.
As 1 mole of \(CuCl_2\) give = 1 mole of \(ZnCl_2\)
Thus 0.052 moles of \(CuCl_2\) give =\(\frac{1}{1}\times 0.052=0.052moles\) of \(ZnCl_2\)
Mass of \(ZnCl_2=moles\times {\text {Molar mass}}=0.052moles\times 136g/mol=7.07g\)
Thus 7.07 g of \(ZnCl_2\) will be produced from the given masses of both reactants.
0.00401g is dissolved in 675 000ml what is the concentration of this solution in ppm
Answers
Answer:
grams of solute in 1,000,000 mL of solution ... A mass of 0.00401 g of salt is dissolved in 675,000 mL of water.
Explanation:
Which material will feel coldest at room temperature?
A. Plastic
B. Glass
C. Metal
O D. Rubber
Answers
The material that feels coldest at room temperature would be metal. That is option C.
What is Metal?
A metal is defined as the materials that are good conductors of heat and electricity and are known to be solid at room temperature.
The physical properties of metals include the following:
high melting points.good conductors of electricity.good conductors of heat.high density.malleable.ductile.Metal feels cold because it conducts heat extremely well. Since room temperature is lower than your body temperature, metal will quickly absorb the heat from your skin, making it feel cold.
Learn more about metals here:
https://brainly.com/question/28183884
#SPJ1
A 13.58 g sample of a compound contains 8.67 g of iron, Fe, 1.60 g of phosphorus, P, and oxygen, O. Calculate the empirical
formula for the compound.
Answers
Answer:
\(Fe_3PO_4\)
Explanation:
To do this, we find the moles of each element. We get around 0.155 moles of Fe, 0.051 moles of P, and 0.206 moles of O. We then divide each one by the smallest one (which is 0.051 moles of P). We then get 3 for Fe, 1 for P, and 4 for O. This correlates to the empirical formula of the compound.
The empirical formula for the compound is \(Fe_3PO_4\) if a 13.58 g sample of a compound contains 8.67 g of iron, Fe, 1.60 g of phosphorus, P, and oxygen, O.
What is the empirical formula?
An empirical formula tells us the relative ratios of different atoms in a compound.
We need to calculate the number of moles:
Given data:
Mass of iron - 8.67 g
Mass of phosphorus -1.60 g
Mass of oxygen -3.31 g
Moles of iron - \(\frac{mass}{molar \;mass}\)
Moles of iron - \(\frac{8.67 g}{56 g/mol}\)
0.15 mole
Moles of phosphorus \(-\frac{mass}{molar \;mass}\)
Moles of phosphorus - \(\frac{1.60 g}{31 g/mol}\)
0.051 moles
Moles of oxygen -\(\frac{mass}{molar \;mass}\)
Moles of oxygen - \(\frac{3.31 g}{16 g/mol}\)
0.20 moles
Dividing each mole using the smallest number that is divided by 0.051 moles.
Fe:P:O :: 3:1:4
The empirical formula for the compound is \(Fe_3PO_4\).
Learn more about empirical formula here:
brainly.com/question/14044066
#SPJ1
What is SO2 shape name?
Answers
Answer:Molecular Formula SO2
Hybridization Type sp2
Bond Angle 119o
Geometry V-Shaped or Bent
Explanation:
hope this helped <3
Identify the missing coefficient in the balanced equation and classify the type of reaction.
Al2(CO3)3 ⟶ Al2O3 + ___CO2
1; Combination
1; Decomposition
3; Combination
3; Decomposition
Answers
Answer:
Explanation:
Part A
Balancing means that the same number of elements on one side of the equation must be the same as the number of elements on the other side.
You need 3 carbons on the right side because right now there is only one. Try putting a 3 in front of the CO2 and see if the oxygens come into balance as well.
Al2(CO3)3 ⟶ Al2O3 + ___CO2
Al2(CO3)3 ⟶ Al2O3 + 3CO2
Balance check
Element Left Right
Al 2 2
Carbon(C) 3 3
Oxygen 9 3 + 6 = 9
And it is balanced.
Part B
It's a decomposition of some kind. Two smaller molecules are created from one larger one.
Why is the classification species not considered a group? (1 point)
O Each species is a separate type of organism.
O Each species is an individual organism.
O Each species lacks the characteristics of the levels above.
O Each species shares characteristics with other species.
Answers
Each species is a separate type of organism.
A species is a group of creatures that share similar traits. The same species of organisms are capable of sexual reproduction as well as interbreeding and producing fertile offspring. It is a fundamental unit of taxonomy and classification.The system is divided into seven categories: Kingdom, Phylum or Division, Class, Order, Family, Genus, and Species. Kingdom is the most inclusive category.In a group, many types of an organism can be included even if they do not share the same traits. But species is a group of organisms that share similar traits.For example, human beings are species as they are all alike in physical features, way of reproduction, etc. But the animal is considered a group because it included a variety of living beings.Therefore, Each species is not considered a group.
Learn more about taxonomy here:
https://brainly.com/question/1304906
#SPJ9
If you had 0.08841 mol of sucrose present in a 625 mL aqueous solution, what would be the molarity of the solution? (Remember that molarity is defined in terms of liters of the solution!)
Answers
The molarity of the solution with 0.08841 moles of sucrose in 625 mL of aqueous solution is 0.1414 M.
Given the number of moles of sucrose (n) = 0.08841mol
The volume of aqueous sucrose solution (V) = 625mL = 0.625L
Let the molarity of solution = M
Molarity (M) is the number of moles of solute per liter of solution. To find the molarity of a solution, we need to divide the number of moles of solute by the number of liters of solution.
Therefore, the molarity of the solution with 0.08841 moles of sucrose in 625 mL of aqueous solution can be calculated as follows:
Molarity (M) = (moles of solute(n)) / (Volume of solution(V))
M = (0.08841 mol sucrose) / (0.625 L solution)
M = 0.1414M sucrose
To learn more about molarity click here https://brainly.com/question/8732513
#SPJ1
Which factor causes Earth’s seasons?
Answers
Answer:
The sun's position near earth
Explanation:
20. Stoichiometry is based on
A. molecular weight.
B. temperature.
C. conservation of matter.
D. pressure.
Answers
Answer:
The correct option is (c)
Answer:
the law of conservation of mass
Explain the role that gravitation played in the formation of the Moon after the large planetesimal hit Earth.
Answers
Answer:
The gravity pulled the bits back and formed a ball which is the moon
Explanation:
hope this helps :)
Answer:
While some of the debris from the collision merged back into Earth, the gravity caused the accretion of much of the debris into a separate ball, forming the Moon.
Explanation:
Consider the following reaction:
Mg(s) + 2HCl(aq)
->
MgCl2 (aq) +H2(8)
If 0.475 g Mg reacts with 124.95 mL of 1.08 M HCl, how many mol of H2 gas will be produced?
Calculate the initial moles of Mg and HCl and find the limiting reactant?
Answers
Answer:The balanced equation of Mg(s) + 2HCl(aq) ==> MgCl2(aq) + H2(g) does NOT tell you how many moles of Mg or H2 are present. It only tells you the MOLE RATIO of the reactants and products. Thus, 1 mole Mg reacts with 2 moles HCl to produce 1 mole of hydrogen gas.
Explanation:The balanced equation of Mg(s) + 2HCl(aq) ==> MgCl2(aq) + H2(g) does NOT tell you how many moles of Mg or H2 are present. It only tells you the MOLE RATIO of the reactants and products. Thus, 1 mole Mg reacts with 2 moles HCl to produce 1 mole of hydrogen gas.
Here, the limiting reactant is Mg. The number of moles of Mg is 0.019 and that of HCl is 0.134. The number of moles of H2 produced is 0.019.
What is limiting reactant ?The limiting reactant in a reaction is the reactant which is fewer in moles and thus, determine the yield of the reaction. In the given reaction, two moles of HCl reacts with one mole of magnesium metal.
Given the mass of Mg = 0.475 g
atomic mass = 24 g/mol
no.of moles = 0.475 /24 = 0.124
volume of HCl = 0.124 L
molarity = 1.08 M
number of moles = molarity × volume = 0.134
The limiting reactant here is magnesium. One mole of Mg reacts with 2 moles of HCl giving 1 mole of hydrogen gas. Then 0.019 moles will give 0.019 moles of hydrogen gas.
Find more on limiting reactant:
https://brainly.com/question/14225536
#SPJ2
The meaning of the word symptom:
Answers
The word "symptom" refers to a specific manifestation or indication of a condition, disease, or disorder that is experienced or observed by an individual.
Symptoms are subjective or objective changes in the body's normal functioning that may be recognized as abnormal, uncomfortable, or problematic. Symptoms can manifest in various ways depending on the nature of the underlying condition. They can be physical, such as pain, rash, cough, fever, or fatigue, indicating an illness or injury affecting the body. Symptoms can also be psychological, such as anxiety, depression, or confusion, reflecting disturbances in mental health.
Symptoms serve as important clues for medical professionals to identify and diagnose diseases or disorders. They provide valuable information about the nature, severity, and progression of an illness, helping healthcare providers formulate appropriate treatment plans. Additionally, symptoms may also be important for individuals to self-assess their own health status and seek appropriate medical attention.
It is essential to note that symptoms alone may not provide a definitive diagnosis, as they can overlap across different conditions. Further evaluation, including medical tests and examinations, is often necessary to confirm a diagnosis and determine the appropriate course of action.
for more such questions on symptom
https://brainly.com/question/21078887
#SPJ8
In order to make observations, an observer must always use what?
Sciene is the subject
Answers
Answer:
In order for a scientist to make observations. They must uses their senses.
Explanation:
The senses include:
Sight
Touch
Smell
Taste
Hearing
burning 12g of urea raise temp of water by 30C what is the enthalpy of combustion for 1kg urea
Answers
The enthalpy of combustion for 1kg of urea is -1223525.84 J/mol.
Urea is a compound that is used in fertilizers and in some plastics.The enthalpy of combustion for urea is the amount of energy that is released when urea is burned. In order to calculate the enthalpy of combustion for 1kg of urea, we need to use the information that is provided to us in the question. Let us start by writing down the balanced equation for the combustion of urea: CO(NH2)2 + 3/2 O2 → CO2 + 2H2O + N2
The balanced equation shows that 1 mole of urea reacts with 1.5 moles of oxygen gas to produce 1 mole of carbon dioxide, 2 moles of water, and 1 mole of nitrogen gas. The enthalpy change for this reaction is equal to the amount of energy that is released when 1 mole of urea is burned.
The heat of combustion (ΔHc) of urea is -632.6 kJ/mol. This means that 632.6 kJ of energy is released when 1 mole of urea is burned. We know that 12g of urea raised the temperature of water by 30°C. We can use this information to calculate the amount of energy that was released when 12g of urea was burned.
The specific heat capacity of water is 4.18 J/g°C. This means that it takes 4.18 J of energy to raise the temperature of 1 gram of water by 1°C. Therefore, it takes 4.18 x 1000 = 4180 J of energy to raise the temperature of 1 kg of water by 1°C.
We know that 12g of urea raised the temperature of water by 30°C. Therefore, the amount of energy that was released when 12g of urea was burned is:
Energy = mass x specific heat capacity x temperature change
Energy = 0.012 kg x 4180 J/kg°C x 30°C
Energy = 1497.6 J
We can now use this information to calculate the enthalpy of combustion for 1kg of urea:
Enthalpy of combustion = energy released / moles of urea burned
Enthalpy of combustion = 1497.6 J / (0.012 kg / 60.06 g/mol)
Enthalpy of combustion = - 1223525.84 J/mol
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
WHat is zinc please answer correct really important
Answers
Answer:
Zinc helps your immune system
Explanation:
What happens when two cars converge
Answers
The binding of glucose to hexokinase O a. is an example of induced-fit binding of a substrate to the active site of an enzyme. b. is not well characterized. O c. is an example of lock-and-key binding of a substrate to the active site of an enzyme. d. differs from the binding of substrates to other kinases.
Answers
The binding of glucose to hexokinase O is an example of lock-and-key binding of a substrate to the active site of an enzyme.
What is lock-and-key binding?
Lock-and-key binding is a type of macromolecular recognition in which the shape of a molecule, known as the "key", fits into the shape of a receptor molecule, known as the "lock". This type of binding is based on the complementary shapes of the two molecules and the weak interactions between them. The key molecule usually binds to the lock molecule with a high degree of specificity and affinity, meaning that the key will only fit into its corresponding lock. This type of binding is essential for many biological processes such as enzyme catalysis and signal transduction.
To learn more about lock-and-key binding
https://brainly.com/question/7197043
#SPJ4
What is the percent of C in Ca(C2H302)2? (Ca = 40.08 gkmol, C = 12.01 g/mol, H= 1.01 g/mol, O = 16.00 g/mol) [?1%C Round your answer to the hundredths place. [?] % C
Answers
Answer:
Ca(C2H3O2)2 has 30.41% carbon by volume
Explanation:
Chemistyd
By mistake you and sat instead of sugar to the
of How can you remove the salt
Answers
If you accidentally add salt instead of sugar to a recipe, you can use vinegar to counteract the salty taste.
If you have added salt instead of sugar to a recipe, then you can try to remove the salt by adding a substance that will counteract its flavor. One such substance is vinegar, which is an acid and can help to neutralize the salty taste. Here are the steps to remove salt from a dish:
1. Remove as much of the salty liquid or sauce as possible.
2. Dilute the remaining sauce or liquid by adding more of the ingredients in the recipe, except for the salt
3. Taste the dish and add more sugar if needed.
4. If the dish is still too salty, add a little bit of vinegar.
5. Keep tasting the dish and adjusting the sugar and vinegar until it is no longer too salty.6. If the dish becomes too sweet, add more of the other ingredients to balance it out.
Know more about salt here:
https://brainly.com/question/20835655
#SPJ8
Calculate the pH of a solution with [H+] = 2.52 x 10^-5.
Answers
Answer:
pH = 4.6
Explanation:
pH is the negative of the log of the hydrogen ion concentration
- log { 2.52 x 10^-5) = ~ 4.6
Determine the rate of the reaction shown directly below if the rate constant k is 1.1 x 10^–2 M^–2 s^–1, the NO concentration is 5.0 x 10^–4 M, and the H2 concentration of 8.0 x 10^–2 M
Thank you! :)
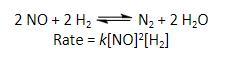
Answers
Answer:
1.76×10^-11
Explanation:
Note: Rate=k[NO]²[H2]² because we have 2mole of hydrogen not 1mole from the equation
R=1.1×10^-2×(5.0×10^-4)²×(8.0×10^-2)²
R=1.1×10^-2×25×10^-8×64×10^-4
R=1.1×25×64×10^-2-8-4
R=1760×10^-14
R=1.76×10^-11
hope it's clear??
What is the pressure exerted by a force of 14.7 N over an area of 0.00482 m²?
Answers
Answer:
\(p= \frac{F}{A} = \frac{ 14.7 }{0.00482} = 3050 \: Pa\)