what is the correct cell notation for a voltaic cell based on the reaction below? cu2 (aq) fe(s) –––> cu(s) fe2 (aq)
Answers
The correct cell notation for a voltaic cell based on the given reaction \(cu2 (aq) + Fe(s) → Cu(s) + Fe2 (aq)\) is as follows chemical reactions in an electrochemical cell.
The cell notation consists of two vertical lines indicating the phase boundary between the two electrodes and a double vertical line that indicates a salt bridge or a porous membrane. In the given chemical reaction, Cu is reduced and Fe is oxidized. Therefore, Cu electrode will be the cathode, where reduction will take place, and Fe electrode will be the anode, where oxidation will take place. The salt bridge or porous membrane will be used to maintain electrical neutrality. In the cell notation, the left-hand side represents the cathode, and the right-hand side represents the anode.
Therefore, the correct cell notation for the given chemical reaction is \(Cu(s)|Cu2+ (aq) || Fe2+ (aq) | Fe(s)\)
This cell notation shows that a copper electrode with copper ions is present on the left side of the cell, and an iron electrode with iron ions is present on the right side of the cell. The double vertical line represents the salt bridge used to complete the circuit.
To know more about chemical reactions visit:
https://brainly.com/question/28792948
#SPJ11
Related Questions
Why are sodium and chlorine the largest dissolved components in ocean water? What is the most abundant dissolved gas in ocean water?
Answers
Sodium (Na) and chlorine (Cl) are the largest dissolved components in ocean water due to the abundance of sodium and chloride ions in the Earth's crust and the continuous input of these elements into the oceans through various processes. Sodium is one of the most common elements in the Earth's crust, and chlorine is widely distributed in rocks, minerals, and salts.
Over millions of years, weathering of rocks, volcanic activity, and erosion release these elements into rivers and ultimately into the oceans. The combination of sodium and chlorine ions results in the formation of sodium chloride, which is commonly known as table salt and contributes to the salinity of seawater.
The most abundant dissolved gas in ocean water is carbon dioxide (CO2). Carbon dioxide dissolves in the surface waters of the ocean through gas exchange with the atmosphere. It plays a crucial role in regulating the pH of seawater and is an essential component of the carbon cycle. Carbon dioxide is involved in various biological and chemical processes in the ocean, including photosynthesis by marine plants and the formation of calcium carbonate shells by marine organisms. Additionally, the increase in atmospheric carbon dioxide due to human activities has led to ocean acidification, which is a significant concern for marine ecosystems.
To know more about Sodium (Na) and chlorine (Cl), click here, https://brainly.com/question/5319005
#SPJ11
Due to the fact that they combine to form the ionic compound sodium chloride (NaCl), also known as salt, sodium (Na) and chlorine (Cl) are the two most abundant dissolved elements in ocean water.
Thus, Salts are among the many dissolved compounds that water from rivers and streams transports into the ocean.
In particular, sodium and chloride ions have accumulated in the ocean throughout time, leading to the high concentration of these elements in seawater. Magnesium, calcium, potassium, and sulphate ions are among the other dissolved substances in ocean water.
Oxygen is the dissolved gas that is most prevalent in ocean water.
Thus, Due to the fact that they combine to form the ionic compound sodium chloride (NaCl), also known as salt, sodium (Na) and chlorine (Cl) are the two most abundant dissolved elements in ocean water.
Learn more about Ocean, refer to the link:
https://brainly.com/question/12738467
#SPJ4
Give the structure that corresponds to the following molecular formula and H1 NMR spectrum: C7H12Cl2 : δ 1.07 (integral = 6203, s); δ 2.28 (integral = 1402, d, J = 6 Hz); δ 5.77 (integral = 700, t, J = 6 Hz) ppm. Note: The integrals are given in arbitrary units.
Answers
Along with the molecular formula C7H12Cl2, a possible structure could be a 1,1,3,3-tetrachlorocyclohexane compound with a long carbon chain, two chlorine atoms, and possibly a double bond or an aromatic system.
The H1 NMR spectrum provides information about the hydrogen atoms in the molecule and their chemical environment. From the spectrum, we can identify three distinct peaks at different chemical shift values.
The peak at δ 1.07 ppm corresponds to hydrogen atoms in a saturated carbon environment. The integral value of 6203 suggests a large number of hydrogen atoms in this environment, indicating a long chain or multiple instances of this group.
The peak at δ 2.28 ppm corresponds to hydrogen atoms in a slightly more deshielded environment, likely adjacent to an electronegative atom or a double bond. The integral value of 1402 suggests a smaller number of hydrogen atoms in this environment.
The peak at δ 5.77 ppm corresponds to hydrogen atoms in a more shielded environment, possibly near a double bond or an aromatic system. The integral value of 700 suggests a smaller number of hydrogen atoms in this environment as well.
The specific arrangement and connectivity of the atoms would require further analysis and experimental data to determine with certainty.
Learn more about H1 NMR spectrum here
https://brainly.com/question/31040944
#SPJ11
what are some careers that require knowledge and use of circuits
Answers
Answer:
Career Information for Jobs that Involve Electricity
Electricians. ...
Line Installers and Repairers. ...
Electrical and Electronics Engineers. ...
Construction Managers. ...
Power Plant Operators, Distributors and Dispatchers. ...
Cardiovascular Technologists and Technicians
Is NaOH a strong base?
Answers
Yes, NaOH, is a strong base.
NaOH, also known as sodium hydroxide
In aqueous solutions, it fully dissociates to form hydroxide ions (OH-). Strong bases have a high degree of dissociation, meaning they break apart completely in water to form their respective ions.
In contrast, weak bases only partially dissociate in water. Strong bases have a pH greater than 7, and NaOH has a pH of around 13.
Strong bases are also highly alkaline and can cause chemical burns if handled improperly. They are commonly used in industrial applications such as soap making, paper production, and oil refining.
A strong base is a substance that ionizes completely or almost completely in an aqueous solution. It releases hydroxide ions (OH-), which increases the hydroxide ion concentration and raises the pH of the solution. Strong bases have a high degree of dissociation, meaning they break apart completely in water to form their respective ions.
To learn more about strong base refer here
https://brainly.com/question/16749233
#SPJ11
Formamide decomposes at high temperature. If 0.186 mol of formamide (HCONH2) dissociates in a 2.16 L flask at 400 K, what are the concentrations of all species present at equilibrium at 400 K? (hint: calculate concentrations first) (b) What is the total pressure in the container at equilibrium?
Answers
Answer:
a) [COHNH₂] = 0.001 mol/L, [NH₃] = [CO] = 0.085 mol/L
b) 5.59 atm
Explanation:
a) The decomposition reaction of formamide is the following:
COHNH₂(d) ⇆ NH₃(g) + CO(g)
The equilibrium constant of the reaction above is:
\(K_{c} = \frac{[NH_{3}][CO]}{[COHNH_{2}]} = 4.84 (400 K)\)
The initial concentration of formamide is:
\( C_{COHNH_{2}} = \frac{\eta}{V} = \frac{0.186 moles}{2.16 L} = 0.086 mol/L \)
Where: η is the number of moles and V is the volume
Now, in the equilibrium the concentration of all species is:
COHNH₂(d) ⇆ NH₃(g) + CO(g)
0.086 - x x x
\( K_{c} = \frac{[NH_{3}][CO]}{[COHNH_{2}]} = \frac{x*x}{0.086 - x} \)
\( 4.84*(0.086 - x) -x^{2} = 0 \)
By solving the above equation for x we have:
x = 0.085 mol/L = [NH₃] = [CO]
[COHNH₂] = 0.086 - 0.085 = 0.001 mol/L
Therefore, the concentrations of all species present at equilibrium at 400 K is [NH₃] = [CO] = 0.085 mol/L and [COHNH₂] = 0.001 mol/L.
b) To find the total pressure in the container we need to find first the constant Kp as follows:
\( K_{p} = K_{c}*RT^{\Delta n} \)
Where R is the gas constant = 0.082 Latm/(Kmol), T is the temperature = 400 K and Δn = 1
\( K_{p} = K_{c}*RT^{\Delta n} = 4.84*(0.082*400)^{1} = 158.8 \)
Now, the total pressure is:
\( p_{T} = p_{COHNH_{2}} + p_{NH_{3}} + p_{CO} \)
The pressure of COHNH₂ can be found using Ideal Gas Law:
\( P = \frac{nRT}{V} = \frac{0.186 moles*0.082 L*atm/(K*mol)*400 K}{2.16 L} = 2.82 atm \)
Using the equilibrium constant we can find the pressure of NH₃ and CO:
COHNH₂(d) ⇆ NH₃(g) + CO(g)
2.82 - x x x
\( K_{p} = \frac{P_{NH_{3}}*P_{CO}}{P_{COHNH_{2}}} \)
\( 158.8*(2.82 - x) - x^{2} = 0 \)
By solving the above equation for x we have:
\( x = P_{NH_{3}} = P_{CO} = 2.77 atm \)
\( P_{COHNH_{2}} = 2.82 - 2.77 = 0.05 atm \)
Thus, the total pressure is:
\( p_{T} = p_{COHNH_{2}} + p_{NH_{3}} + p_{CO} = (0.05 + 2.77 + 2.77) atm = 5.59 atm \)
Hence, the total pressure in the container at equilibrium is 5.59 atm.
I hope it helps you!
2. Conduction occurs best in metals because they have a high
Answers
The correct answer is when electrons bump into atoms and other electrons.
Because of how tightly packed their particles are, metals are particularly good heat conductors because vibrations are transferred very fast. Additionally, they have a lot of free electrons.
Metals get their strength and other qualities from these when they gently float through the framework. The free electrons that are nearest to the heat source are heated as the metal warms up. As a result, they move more quickly through the metal, crashing into other electrons as well as atoms. They naturally vibrate more quickly as a result (or move through the metal faster in the case of collisions with other free electrons). As a result, the metal swiftly dissipates the heat.
To learn more about conduction refer the link:
https://brainly.com/question/21496559
#SPJ9
Calculate the ph of a solution prepared by mixing 20.0 ml of 0.30 m hcl with 15.0 ml of 0.60 m naoh
Answers
The pH of the solution prepared by mixing 20.0 mL of 0.30 M HCl with 15.0 mL of 0.60 M NaOH is 1.96 .
To calculate the pH, we need to determine the concentration of the resulting solution after the mixing of the acid and base.
First, we determine the moles of HCl and NaOH used in the solution:
Moles of HCl = Volume of HCl (L) * Concentration of HCl (M)
= 0.020 L * 0.30 M
= 0.006 mol
Moles of NaOH = Volume of NaOH (L) * Concentration of NaOH (M)
= 0.015 L * 0.60 M
= 0.009 mol
Since HCl and NaOH react in a 1:1 ratio, the moles of HCl used in the reaction are equal to the moles of NaOH used. Therefore, the excess moles of HCl remaining in the solution are:
Excess moles of HCl = Moles of HCl - Moles of NaOH
= 0.006 mol - 0.006 mol
= 0.000 mol
The excess moles of HCl do not contribute to the solution's pH, as they are neutralized by the NaOH. Thus, the remaining solution is effectively a solution of NaCl, which is a neutral salt.
Therefore, the pH of the solution prepared by mixing HCl and NaOH is 1.96 .
Learn more about pH of the solution from the given link https://brainly.com/question/23857908
#SPJ11
identify the limiting reactant when 7.28 grams of magnesium oxide reacts with 4.50 grams of aluminum to make magnesium and aluminum oxide? i need a typed answer a link wont work
Answers
Answer:
the limiting reactant is aluminum
Explanation:
50 extra points and brainliest if right !!!
Why are there volcanoes on the Ring of Fire?
O A. Radioactive elements decay.
B. There is no plate movement.
C. There are no earthquakes. D. Two plates are colliding.
Answers
Answer:
D. Two Plates are colliding.
Answer:
D
Explanation:
The ring of fire has very large amount of plate collision so it cannot be B.
It can't be A either because the ring of fire has nothing to do with radioactive element decay.
The two main things with the ring of fire is the fact that there are a lot of volcanos and earthquakes, so it cannot be C.
Since the ring of fire has so many collisions of plates, and none of the other answers are correct, the answer is D.
Hope this helps!
how many grams of sodium hydroxide are required to dissolve in 232 g of water to make a 2.88 m solution
Answers
Grams of Sodium hydroxide : 26.72 g
Further explanationMolality shows the number of moles dissolved in every 1000 grams of solvent.
m = n. (1000 / p)
m = Molality
n = Number of moles of solute
p = Solvent mass (gram)
Can be written :
\(\tt m=\dfrac{mol~solute}{kg~solvent}\)
mol solute(NaOH)m=2.88
kg solvent(water)=232 g=0.232 kg
\(\tt mol~solute=m\times kg~solvent\\\\mol~solute=2.88\times 0.232=0.668\)
mass of NaOH\(\tt mass=mol\times MW\\\\mass=0.668\times 40~g/mol=26.72~g\)
please help me
16 1 point What is the decay rate of a sample of Oxygen-21 if the sample has 8.31x1017 atoms and a decay constant of 0.203/s? 4.09x1018Bq 1.69x10¹7Bq 0.203Bq 2.44x10-1⁹Bq Previous
Answers
decay rate of approximately 1.69x10^17 Bq (becquerels),
The decay rate of a radioactive sample is determined by the number of radioactive atoms present and the decay constant, which represents the probability of decay per unit of time.
To calculate the decay rate, we multiply the number of atoms in the sample by the decay constant. In this case, the sample has 8.31x10^17 atoms and a decay constant of 0.203/s. Multiplying these values gives a decay rate of approximately 1.69x10^17 Bq (becquerels), which represents the number of decays per second in the sample.
Learn more about Oxygen here : brainly.com/question/13905823
#SPJ11
what is the least measurement of black hole,

Answers
Answer:
Here is the correct answerblack hole in ourter space for the first time the scientific research
and development of the following layers lethargic
Explanation:
STUDY CORRECTION.
What properties could be used to describe an atom of a specific element?
Answers
There are two properties that can be used to identify an element: the atomic number or the number of protons in an atom. The number of neutrons and number of electrons are frequently equal to the number of protons, but can vary depending on the atom in question.
Why did early people most likely choose to settle in Mesopotamia?
Answers
Answer:
People wanted to settle there because it had good living conditions. There was rich soil, and lots of water sources.
I hope this helps!
Describe the relationship between the solubility rate of a solid and temperature.
Answers
Answer:
hope you like
Explanation:
the solubility rate of a solid is directly proportional to the temperature. if the temperature is high the solubility will also increase
What is the number of copper atoms in a unit cell of a face-centered cubic lattice of copper?
Answers
In a face-centered cubic (FCC) lattice, each unit cell has 8 corner atoms and 1 center atom. Thus, a unit cell of a FCC lattice of copper would contain 8 corner copper atoms and 1 center copper atom, giving a total of 8 + 1 = 9 copper atoms in the unit cell.
In a face-centered cubic (FCC) lattice of copper, the unit cell is made up of 8 corner atoms and a single atom in the center. Each corner atom of the unit cell is shared with 8 other unit cells and the center atom is shared with a total of 8 unit cells. Therefore, the number of copper atoms in a unit cell of an FCC lattice of copper is 8 corner atoms + 1 center atom = 9 atoms. This is a basic characteristic of FCC structures, which are very commonly found in metals and are known for their high packing efficiency and close-packed arrangement of atoms.
Learn more about Copper here: brainly.com/question/13677872
#SPJ4
Question 8 (1 point) 2H₂ + O₂ O2 --> 2 H20 Molar mass of H2 = 2.0 O2 =32.0 H2O=18.0 If you start with 20 grams of Oz, how many moles of H2O can be made? 1.25 mol 320 mol 1280 mol 0.31 mol Question 9 (1 point)
Answers
Answer:
the moles of H2O are equal to 1.25mol
I hope this help
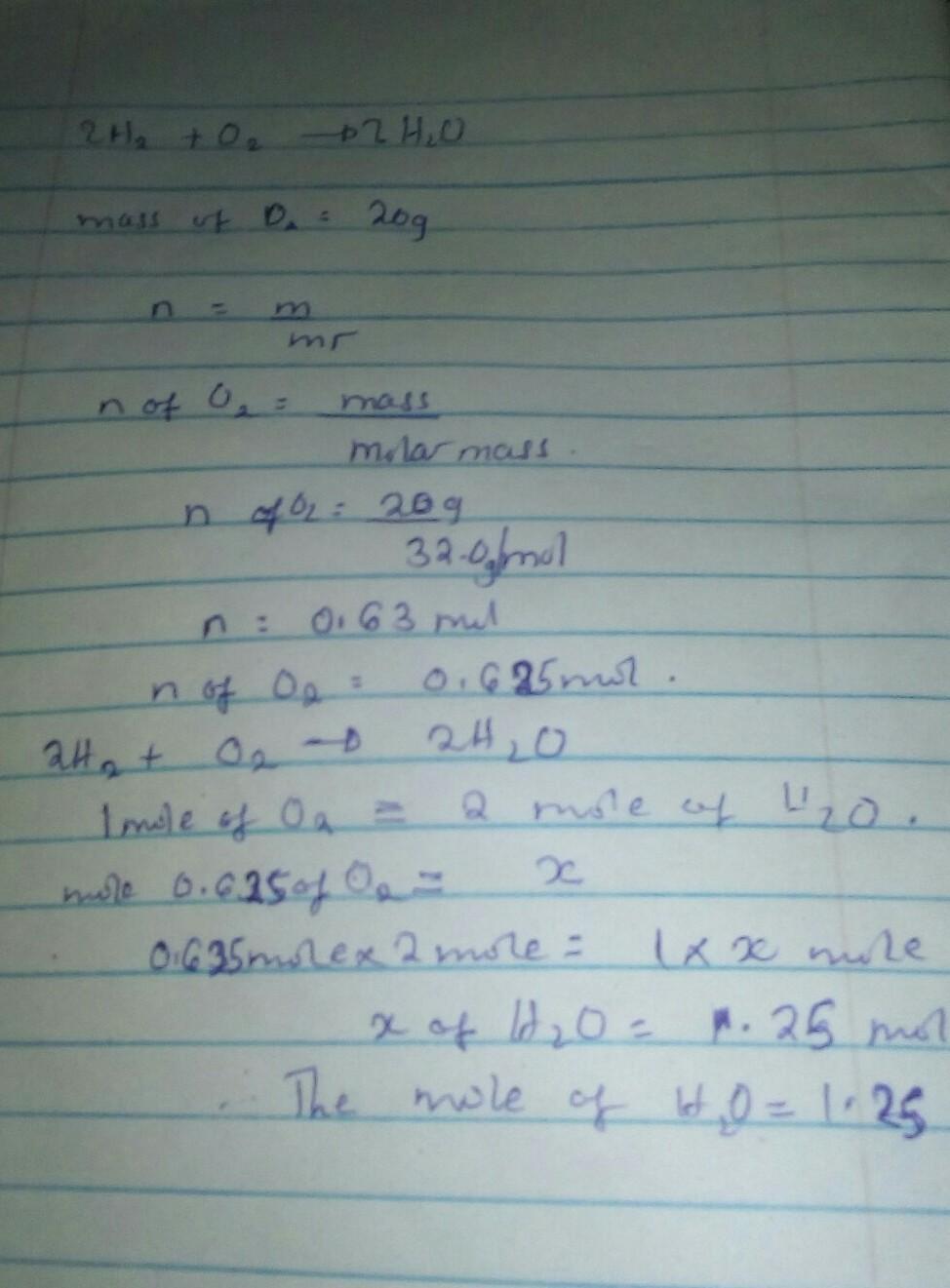
Answer:
f
Explanation:
A sample of platinum has a mass of 16.1 g and a volume of 0.75 cm^3. What is the density of platinum?
Answers
21.4666666667 g/cm^3
Explanation:
The density of a substance can be found by dividing the mass by the volume.
d=m/v
The mass of the sample is 16.1 grams and the volume is 0.75 cubic centimeters.
m= 16.1 g
v=0.75 cm^3
Substitute the values into the formula.
d=16.1 g / 0.75 cm^3
Divide
d= 21.4666666667 g/cm^3
The density of platinum is 21.4666666667 grams per cubic centimeter.
choc When heated in the presence of an acid, a triglyceride produced a linoleic acid residue, a palmitoleic acid residue, and an oleic acid residue. What is possible structures for this triglyceride
Answers
Based on the given information, the triglyceride could have the following possible structures:
1. Linoleic acid - Palmitoleic acid - Oleic acid
2. Oleic acid - Palmitoleic acid - Linoleic acid
3. Palmitoleic acid - Linoleic acid - Oleic acid
These structures involve the three fatty acid residues produced when the triglyceride is heated in the presence of an acid - linoleic acid, palmitoleic acid, and oleic acid. The order of these residues can vary in different triglycerides.
Hi! To determine the possible structures of the triglyceride that produced a linoleic acid residue, a palmitoleic acid residue, and an oleic acid residue when heated in the presence of an acid, follow these steps:
1. Identify the three fatty acid residues:
- Linoleic acid residue (18:2, meaning 18 carbons and 2 double bonds)
- Palmitoleic acid residue (16:1, meaning 16 carbons and 1 double bond)
- Oleic acid residue (18:1, meaning 18 carbons and 1 double bond)
2. Recognize that triglycerides are composed of a glycerol molecule (with 3 hydroxyl groups) esterified with three fatty acid residues.
3. Attach the three fatty acid residues to the glycerol molecule in different combinations.
Your answer: Possible structures for this triglyceride include different combinations of a glycerol molecule esterified with a linoleic acid residue, a palmitoleic acid residue, and an oleic acid residue.
to know more about triglycerides here:
brainly.com/question/5096426
#SPJ11
to ensure that the decolorization of crystal violet reaction takes place within the linear range of the crystal violet calibration curve, and that there is an excess of hydroxide ions, 10.0 ml of the stock solution of crystal violet is diluted twice. first, with 10.0e so ml of distilled water and then with 10.0 ml of 0.10 m naoh. what is the [oh-] in the final dilute solution?
Answers
A calibration curve is a regression model that uses the instrument's reaction to known standards to forecast the unknown concentrations of interest-related analytes.
How do you calculate calibration curve?
The formula will have the general form of y = mx + b, where m denotes the slope and b the y-intercept, as in y = 1.05x + 0.2.When making adjustments to measurements made on samples whose values are unknown, use the equation for the calibration curve.Solve for y (the "true" value) by substituting the measured value (x) into the equation. We have four milliliters of a 2.5 x 10–5 molar solution of crystal violet.the crystal violet kinetics process, too.Usually, adding sodium hydroxide in much larger amounts causes the violet color to fade.You must now add the two of them together to complete this reaction.However, we're attempting to determine the crystal violet concentration prior to the reaction.Therefore, we are not attempting any story geometry; rather, we are attempting to determine the concentration of crystal violet at the start of the reaction.And it's not the crystal violet's concentration.We have four milliliters of a crystal violet solution that is 2.5 x 10 n-5 molar in size.When doing a crystal violet kinetics reaction.The violet tint typically vanishes as you add sodium hydroxide in much bigger amounts.Currently, you need to add the two together in this particular reaction.However, we're looking for the crystal violet concentration just before the reaction starts.We are only attempting to determine the concentration of the crystal violet at the start of the reaction; we are not attempting to perform any stories geometry here.Furthermore, it is not the crystal violet's concentration.All of the components in that particular reaction become diluted when two substances are mixed together.So, when you combine two milliliters of sodium hydroxide solution with four milliliters of the crystal violet solution.The total of those volumes makes up your new volume.Therefore, the result of mixing them is a six-ml solution.Therefore, we must apply the equation polarity times volume is equivalent to mill, aren? T times volume here in order to get the concentration before any of the sodium hydroxide reacts.The number of moles remains constant because we haven't initiated the reaction yet, and it also remains constant in this location.We therefore have a 2.5 times 10 to 5 molar solution. Therefore, we have a 2.5 x 10-5 molar solution in a four milliliter volume.We are currently working on the new mill arat E.However, we are aware that the current volume of our system is six mL.We shall divide both sides by six to obtain the new milliarat E, and we will discover that the new polarity is 1.67 times 10 to the -5moles per liter.We diluted the crystal violet with our other solutions, which reduced the polarity.
To learn more about calibration curve refer
https://brainly.com/question/13492847
#SPJ4
PREPARATION OF BASES
Answers
The preparation of bases involves several methods that are used to create substances with basic or alkaline properties are Reaction of metal with water, Reaction of metal oxide with water, Neutralization reaction, Ammonia gas dissolving in water and Partial neutralization of a strong base with a weak acid.
Reaction of metal with water: Certain metals, such as sodium or potassium, react with water to form hydroxides. For example, sodium reacts with water to produce sodium hydroxide (NaOH).
Reaction of metal oxide with water: Metal oxides, such as calcium oxide (CaO) or magnesium oxide (MgO), can be added to water to form metal hydroxides. This process is known as hydration. For instance, when calcium oxide reacts with water, it forms calcium hydroxide (Ca(OH)2).
Neutralization reaction: Bases can be prepared by neutralizing an acid with an appropriate alkaline substance. This involves combining an acid with a base to form water and a salt. For example, mixing hydrochloric acid (HCl) with sodium hydroxide (NaOH) results in the formation of water and sodium chloride (NaCl).
Ammonia gas dissolving in water: Ammonia gas (NH3) can dissolve in water to form ammonium hydroxide (NH4OH), which is a weak base.
Partial neutralization of a strong base with a weak acid: Mixing a strong base, such as sodium hydroxide (NaOH), with a weak acid, like acetic acid (CH3COOH), results in the formation of a base with a lesser degree of alkalinity.
These methods are utilized in laboratories, industries, and various applications where bases are required, such as in the production of cleaning agents, pharmaceuticals, and chemical reactions. Each method has its own advantages and specific applications depending on the desired base and its properties.
The question was incomplete. find the full content below:
What are the various methods involved in the preparation of bases?
Know more about Neutralization Reaction here:
https://brainly.com/question/23008798
#SPJ8
Gallium changes it’s state of matter from solid to liquid in someone’s hand. Think about other substances that you are familiar with that change state. Ice melts in the Sun, and soup steams when it boils. Are you starting to get some ideas on why materials change state? Use gallium as an example to make a claim about what causes a substance d to change states
CLAIM
Gallium changes state because…
Answers
A matter can change from one state to another by absorbing or losing energy. Some of the example of such changes are melting, boiling, freezing, etc. Here 'Ga' changes into liquid state at high temperature.
What are states of matter?The three states of matter represents the three distinct physical forms in which matter can take in most environments. The common states of matter are solid, liquid and gas. A change of state is a physical change in the matter.
When the temperature or pressure of a system changes, then there occurs a change of state. The intermolecular interaction between the molecules increases with the increase in temperature and pressure. Similarly when the temperature decreases, molecules form a rigid structure.
Thus a change of state occurs on changing some parameters.
To know more about states of matter, visit;
https://brainly.com/question/9402776
#SPJ1
A very disorganized scientist has lost track of some data from a recent experiment and can no longer determine what element she was working with. Now, she refers to it as element X. She knows the element has a smaller atomic radius than strontium, a larger ionization energy than phosphorus, and a smaller electronegativity than chlorine. What is the likely identity of element X?
Answers
Answer :
The element X which ha a smaller atomic radius than strontium , a larger ionization energy than phosphorous , and a smaller elctronegativity than chlorine is Nitrogen
Explanation:
Here are some properties of Nitrogen-
Atomic number of Nitrogen is 7 Atomic mass of nitrogen is 14.0067 g.mol -1Nitrogen is a popular non-metal gas that is usually colourless, odourless, tasteless and often diatomic. In its outer shell, it has five electrons, but in most compounds, it is trivalent. Applications- In the manufacture of ammonia, the greatest single commercial use of nitrogen is as a part, subsequently used as fertilizer and to produce nitric acid. Liquid nitrogen (often referred to as LN2) is used as a refrigerant for the freezing and transport of food items, for the protection of bodies and for the reproduction of food products. Nitrogen is a constituent in all living tissues and comprises 78 percent of the Earth 's atmosphere. Nitrogen, since it is a component of DNA and, as such, is part of the genetic code, is an integral element of life.Hence , the X element is NITROGEN.
PLEASE HELP ILL GIVE BRAINLYIST
In which domain do organisms live deep in the Pacific Ocean where hot gases and molten rock spew from vents on the ocean floor?Immersive Reader
bacteria
eukarya
archaea
Answers
Answer:
bacteria
Explanation:
Somebody look at all the science questions i posted plzzz help ( i will give brainliest
Answers
Answer:
ok
Explanation:
In the diatomic molecule HCl, the H and the Cl share a pair of
electrons. By doing so, the hydrogen atom attains the electron
configuration of while chlorine attains the electron
configuration of
Answers
Answer:
helium and argon
Explanation:
The helium dimer is a van der Waals molecule containing two helium atoms with the formula He2. This chemical is the biggest diatomic molecule, which is a molecule made up of two atoms that are bound together. This dimer's connection is so weak that it will break if the molecule spins or vibrates too much.The noble gases (helium, neon, argon, krypton, xenon, and radon) are also monatomic gases at STP. To distinguish them from other gases that are chemical compounds, homonuclear diatomic gases and noble gases are referred to as "elemental gases" or "molecular gases."Calculate the frequency of 4.1 x 10^-19
Answers
What are your initial ideas for what we can do to stop what is occurring to planet earth and ensure the survival of humans and other species?
What can humans do to survive as a species if we are not successful in the negative changes occurring to planet earth?
Answers
Answer: Change the way we run our economy.
Explanation: The way that the world, as a whole, runs its government and economy, is destroying the environment. If we can change this, and find another, better, way to run our countries and cities, the environment will get better, and we won't ruin our planet.
Calculate the specific heat capacity of the unknown metal given the
following information; the mass of the metal is 225 g, the temperature
raises by 13C and the energy absorbed was 1363 joules.
A. 0.466 j/gC
B.1.02j/gC
C.0.96 j/gC
D.2.14 j/gC
Answers
Answer: The specific heat capacity of the unknown metal given is \(0.466 J/g^{o}C\).
Explanation:
Given: Mass = 225 g
Change in temperature = \(13^{o}C\)
Heat energy = 1363 J
The formula used to calculate specific heat is as follows.
\(q = m \times C \times \Delta T\)
where,
q = heat energy
m = mass of substance
C = specific heat
\(\Delta T\) = change in temperature
Substitute the values into above formula as follows.
\(q = m \times C \times \Delta T\\1363 J = 225 g \times C \times 13^{o}C\\C = \frac{1363 J}{225 g \times 13^{o}C}\\= 0.466 J/g^{o}C\)
Thus, we can conclude that the specific heat capacity of the unknown metal given is \(0.466 J/g^{o}C\).
The process that converts straight-chain alkanes into branched hydrocarbons is called A hydrolysis B.cracking C. hydrogenation D. reforming
Answers
The process that converts straight-chain alkanes into branched hydrocarbons is called: B. cracking.
The process that converts straight-chain alkanes into branched hydrocarbons is called option B: cracking. Cracking is a chemical process widely used in the petroleum industry to break down larger hydrocarbon molecules into smaller ones. It involves the thermal decomposition of hydrocarbons under high temperatures and pressures, often in the presence of a catalyst.
During cracking, long-chain alkanes are subjected to heat and pressure, causing the carbon-carbon bonds to break. This results in the formation of shorter hydrocarbon chains, including branched hydrocarbons. The process can occur in various forms, such as thermal cracking, catalytic cracking, or hydrocracking, depending on the specific conditions and desired products.
By converting straight-chain alkanes into branched hydrocarbons, cracking enhances the volatility, stability, and reactivity of the resulting hydrocarbon products. It is an essential process in the production of gasoline, diesel fuel, and other valuable hydrocarbon derivatives, as branched hydrocarbons often exhibit improved combustion characteristics and higher octane ratings.
Overall, cracking plays a vital role in the petroleum refining industry, enabling the transformation of long-chain alkanes into a more diverse range of hydrocarbon products with desired properties and applications.
To know more about alkanes refer here:
https://brainly.com/question/24270289
#SPJ11