Answers
The actual average mass of 1 lithium atom is 6.941 grams/atom this is equal to one mole of lithium.
What is atomic mass ?The fundamental physical property of matter is mass. The atomic mass refers to the mass of an atom or molecule. To calculate the average mass of elements and molecules and to solve stoichiometry problems, the atomic mass is used.
Lithium, for example, has an atomic mass of 6.941 grams, which is equivalent to one mole of lithium. This is why we express atomic and molecular masses in grams per mole, or g/mol.
Thus, The actual average mass of 1 lithium atom is 6.941 grams/atom.
To learn more about the atomic mass, follow the link;
https://brainly.com/question/17067547
#SPJ1
Related Questions
Como es la
fórmula química del agua
Answers
Answer:
h2o
Explanation:
Select the correct answer.
What are factors of production?
OA.
О в.
O c.
OD.
all the human efforts involved in the production process
all the resources used to produce any goods and services
all the physical tools and equipment used in the production process
all the naturally occurring resources found in land, air, and water
Reset
N

Answers
Answer: B. all the resources used to produce any goods and services
Explanation:
The factors of production are all the resources used to produce any goods and services. This means our answer is option B.
This includes, but is not limited to, land, labor, capital, and entrepreneurship.
A car's engine block is made of steel and has a mass of 21080g. How much heat (J) is absorbed by the engine block when its temperature is raised from 20°C to 90°C?
Answers
The heat absorbed by the engine block when its temperature is raised from 20°C to 90°C is 665,640 J.
To calculate the heat absorbed by the engine block, we can use the equation:
Q = mcΔT
where Q is the heat absorbed, m is the mass of the engine block, c is the specific heat capacity of steel, and ΔT is the change in temperature.
First, we need to calculate the specific heat capacity of steel. The specific heat capacity of steel is typically around 0.45 J/g°C.
Using this value and the given values of mass and temperature change, we can calculate the heat absorbed by the engine block as follows:
Q = (21080 g) x (0.45 J/g°C) x (90°C - 20°C)
Q = 21080 g x 0.45 J/g°C x 70°C
Q = 665,640 J
For more question on heat absorbed click on
https://brainly.com/question/30361927
#SPJ11
please help me i need this asp

Answers
how many moles of F are in 708.5g of F4C?
Answers
The number of mole of F present in 708.5 grams of F₄C is 32.2 moles
How do i determine the number of mole of F present 08.5 grams of F₄C?First, we shall determine the mole in 708.5 grams of F₄C. Details below:
Mass of F₄C = 708.5 grams Molar mass of F₄C = 88 g/mol Mole of F₄C =?Mole = mass / molar mass
Mole of F₄C = 708.5 / 88
Mole of F₄C = 8.05 moles
Finally, we shall determine the number of mole of F in 708.5 grams (i.e 8.05 moles) of F₄C. Details below:
From the chemical formula of F₄C,
1 mole of F₄C contains 4 moles of F.
Therefore,
8.05 moles of F₄C will contain = (8.05 mole × 4 mole) / 1 mole = 32.2 moles of F.
Thus, we can conclude from the above calculation that the number of mole of F present is 32.2 moles
Learn more about mole composition:
https://brainly.com/question/21323029
#SPJ1
How many seeds would be in 14 kg of apples?
Given:12
Need to Find: Go?
Useful Equivalencies:
Answers
Answer:
thete are 672 seeds in 14 kg
If a reaction starts with 30 grams of material, how many grams of material should be present at the end, according to the Law of Conservation of Mass.
please help me
Answers
If a reaction starts with 30 grams of material, the same amount of mass 30 grams of material should be present at the end, according to the Law of Conservation of Mass.
The law of conservation of mass states that no atoms are misplaced or made in a chemical reaction. as an alternative, the atoms be a part of collectively in distinctive methods to form merchandise.
The law of conservation of mass states that during a chemical reaction mass is neither created nor destroyed. for example, the carbon atom in coal turns into carbon dioxide when it's miles burned. The carbon atom changes from a stable structure to gas but its mass does not trade.
The law of conservation of mass states that depend cannot be created or destroyed in a chemical response. for example, when wooden burns, the mass of the soot, ashes, and gases equals the unique mass of the charcoal and the oxygen when it first reacted. So the mass of the product equals the mass of the reactant.
Learn more about Conservation of Mass here:-https://brainly.com/question/15289631
#SPJ1
Explain how the processes of photosynthesis and respiration are related to each other
Answers
Answer:
Explanation:
The end product of Photosynthesis is glucose that is used in cellular respiration to make ATP. The glucose is then turned back into carbon dioxide, which is used in photosynthesis. While water is broken down to form oxygen during photosynthesis, in cellular respiration oxygen is combined with hydrogen to form water.
How is steel made from the raw product of the blast furnace known
as "pig iron"? What are the advantages of using steel?
List references used (if any were used) to answer this question.
Answers
Steel is produced from pig iron through a process known as steelmaking or iron and steel production.
The pig iron obtained from the blast furnace contains high amounts of carbon, impurities, and other elements. To convert pig iron into steel, the carbon content needs to be reduced to desired levels, and impurities must be removed.One common method of steelmaking is the basic oxygen process (BOP). In this process, pig iron is placed in a vessel called a converter, where oxygen is blown through the molten metal. The oxygen reacts with the carbon and impurities, causing them to oxidize and form gases that are released. Alloying elements and desired additives can be added at this stage to achieve specific steel properties. Another method is the electric arc furnace (EAF), where an electric arc is used to heat and melt the pig iron, allowing impurities to be oxidized and removed.The advantages of using steel are numerous. Steel is strong, durable, and versatile, making it suitable for a wide range of applications. It has high tensile strength, which means it can withstand heavy loads and pressures. Steel is also resistant to corrosion, making it ideal for construction, infrastructure, and transportation projects. It is a recyclable material, contributing to sustainability and reducing environmental impact. Additionally, steel can be fabricated into various shapes and sizes, allowing for customization and flexibility in design.References:
A. Ghosh and A. Chatterjee, Ironmaking and Steelmaking: Theory and Practice, PHI Learning, 2008.
R.H. Tupkary and V.R. Tupkary, An Introduction to Modern Iron Making, Khanna Publishers, 2010.
J.R. Davis, ed., ASM Specialty Handbook: Carbon and Alloy Steels, ASM International, 1995.
for such more questions on production
https://brainly.com/question/25597694
#SPJ8
Pure water and pure salt are poor conductors of electricity. When salt is dissolved in water, the resulting solution conducts electricity well. Which statement explains why this occurs with these substances?
The process of dissolving makes the electrons in their atoms free to move
The process of dissolving makes the atoms in them free to move
The process of dissolving makes the electrons in their atoms more closely bound
The process of dissolving makes the atoms in them more closely bound to each other
Answers
Answer:the process of dissovlving makes the ecectrons in their atoms more closely bound
Explanation:
thats what my lesson said
The solution of water and salt are good conductor of electricity because the process of dissolving makes the electrons in their atoms free to move.
The correct option is (A).
What makes a solution of water and salt a good conductor?Pure water and salt are bad conductor of electricity.
But the solution of water and salt is a good conductor because when a salt's dissociated it's ions are free to move about in solution when it dissolves, allowing a charge to flow freely.
Because it includes ions, the resulting solution will conduct electricity.
Thus, option (A) the process of dissolving makes the electrons in their atoms free to move, that's why the solution of water and salt are good conductor of electricity.
Learn more about solution, here:
https://brainly.com/question/7932885
Nitrogen is a group 15 element. What does being in this group imply about the structure of the nitrogen atom?
O A. Nitrogen has 15 valence electrons.
OB.
Nitrogen has 15 neutrons.
OC. Nitrogen has 5 valence electrons.
D.
Nitrogen has 5 neutrons.
Answers
Answer:
D. Nitrogen has 5 valence electrons.
Explanation:
Nitrogen is an element in group 5A of the periodic table. Elements in group 5A all contain just 5 valence electrons. (Electrons in the outer shell).
**Elements are organized into these groups in a periodic table based on the number of valence electrons which determines their charge. (Does not apply to transition metals)
Match the atoms to their correct electron configuration.
(Answer choices in photo)

Answers
Answer:
The answer to your questions are given below
Explanation:
The answer to the questions given above can simply be obtained by writing the electronic configuration of each atom.
The electronic configuration of each atoms can be written as follow:
Fluorine, F (9) => 1s² 2s²2p⁵
Sodium, Na (11) => [Ne] 3s¹
Helium, He (2) => 1s²
Calcium, Ca (20) => [Ar] s²
Nitrogen, N (7) => 1s² 2s²2p³
Sulphur, S (16) => [Ne] 3s²3p⁴
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
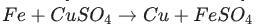
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
Re-read the Topic 2 Learning Activities titled “Glycolysis” and “Overview of Photosynthesis”. What makes these necessary fundamental processes? Use an argument from the reading to support your answer. In what ways are these two processes similar? How are they different?
Answers
Glycolysis and photosynthesis are fundamental processes that are necessary for the survival of living organisms. They are similar in that they both involve the conversion of energy, but differ in the source of energy used, the location of the process, and the requirement for oxygen.
Glycolysis and photosynthesis are two necessary fundamental processes. Glycolysis is a metabolic pathway that occurs in the cytoplasm of cells. The glycolysis process is necessary because it produces ATP, which is the energy required for all cellular activities.
The energy is produced by breaking down glucose into two pyruvate molecules.Photosynthesis is the process by which green plants make their food. During photosynthesis, light energy is converted into chemical energy, which is stored in glucose molecules. This process is also necessary as it provides food and oxygen for most living organisms to survive.In terms of similarities, both glycolysis and photosynthesis are processes that involve the conversion of energy.
In glycolysis, glucose is converted into pyruvate and ATP, while in photosynthesis, light energy is converted into chemical energy. Both processes are also vital to the survival of living organisms.The primary difference between the two processes is the source of energy used. Glycolysis uses glucose as the primary energy source while photosynthesis uses light energy from the sun.
Glycolysis occurs in the cytoplasm of cells while photosynthesis takes place in the chloroplasts of plant cells. Glycolysis is an anaerobic process that does not require oxygen, while photosynthesis is an aerobic process that requires oxygen and releases it as a byproduct.
for more such questions on conversion of energy
https://brainly.com/question/15105485
#SPJ8
HELP ASAP!!! Plzzz
How many total atoms of oxygen are present in the molecules represented here?

Answers
what are 4 molecules made of the same substance
Answers
Answer:
H2O (water)N2 (nitrogen)O3 (ozone)CaO (calcium oxide)Explanation:HOPE IT HELPS
barrier islands are low and narrow sandy islands that form a rim offshore from a coastline. these islands protect inland shores from the surf,especially during storms. theses islands are becoming increasingly developed because people want to live by the open ocean, yet the island themselves are not permaneny. why aren't the islands permanent?
A. People developthe islands and remove sand during housing construction.
B. Offshore earthquakes cause the islands to sink below sea level.
C. The wind and the waves are constantly redistributing the sand.
D. Development companies mine the sand for use in inland construction projects.
Answers
Barrier islands are not permanent because the wind and the waves are constantly redistributing the sand.
What are barrier islands?Barrier islands are particularly flat or lumpy sand regions that are formed parallel to the mainland shore by wave and tide action. They typically appear in groups called chains, which can range in size from a few to more than a dozen islands.
They are called "barriers" because they act as a physical barrier, protecting the mainland from the effects of strong waves, storm surges, and flooding. Barrier islands are formed by a variety of processes, including longshore drift, sediment deposition, and sea level changes.
Barrier islands typically consist of sandy beaches on their seaward side and marshes, lagoons, or dunes on their landward side.
Learn about Barrier islands here https://brainly.com/question/1647030
#SPJ1
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
Please help me do this

Answers
The total mass of the balloon and its content is 1521.17 g, the number of moles of CO₂ in the balloon is 34.15 mol, and the number of CO₂ molecules in the balloon is 2.06 x 10²⁵ molecules.
a) The molar mass of CO₂ is 44.01 g/mol. To find the total mass of the balloon and its content, we need to add the mass of the balloon (20g) to the mass of the CO₂ inside the balloon.
Mass of CO₂ = number of moles of CO₂ x molar mass of CO₂
Since the balloon is at STP (standard temperature and pressure), we can use the molar volume of a gas at STP (22.4 L/mol) to find the number of moles of CO₂ in the balloon:
Volume of CO₂ = Volume of balloon = 765 L (at STP)
Number of moles of CO₂ = volume of CO₂ / molar volume of a gas at STP
= 765 L / 22.4 L/mol
= 34.15 mol
Mass of CO₂ = 34.15 mol x 44.01 g/mol
= 1501.17 g
Total mass of balloon and its content = 20 g + 1501.17 g
= 1521.17 g
b) Number of moles of CO₂ in the balloon is 34.15 mol
c) To find the number of CO₂ molecules in the balloon, we need to use Avogadro's number (6.02 x 10²³ molecules/mol).
Number of CO₂ molecules = number of moles of CO₂ x Avogadro's number
= 34.15 mol x 6.02 x 10²³ molecules/mol
= 2.06 x 10²⁵ molecules
To know more about the Mass, here
https://brainly.com/question/22104139
#SPJ1
What volume of water (H_{2}*O) in milliliters, can vaporize at 100C given 163 kJ of energy? For water, Delta H vap =40.66 kJ mol .
Answers
The volume of the water is obtained as 72 mL.
What is the volume of the water?We know that the heat of vaporization is the heat that is required to raise convert the substance from the liquid state to the gaseous state. Now, we know that we can be able to find the energy from the use of the formula;
H = mL
H = heat that is required
m = Number of moles of the object
L = Heat of vaporization
We know from the problem that we have that;
H = 163 kJ
L = 40.66 kJ/mol
Then we would have;
Number of moles = H/L
= 163kJ/40.66 kJ/mol
= 4 moles
Mass of water = 4 moles * 18 g/mol = 72 g
Since the density of water = 1 g/mL
Volume = Density * mass
= 1 g/mL * 72 g
= 72 mL
Thus, the volume of the water that would be vaporized in the process that we have described is about a volume of 72 mL.
Learn more about vaporization:https://brainly.com/question/14578189
#SPJ1
Predict whether reactants or products will be favored at equilibrium for the below reaction.
Kp= 2.2 x 10^6 at 298K
2COF2 (g) + ⇌ CO2(g) + CF4(g)
Answers
Answer:
The products will be favored at equilibrium.
Explanation:
The balanced chemical equation for the reaction is the following:
2 COF₂ (g) + ⇌ CO₂(g) + CF₄(g)
The reactant is COF₂ (left side) and the products are CO₂ and CF₄ (right side).
The equilibrium constant is given by the ratio between the partial pressures (P) of products and reactants, because they are in the gas phase. Thus, the expression of the equilibrium constant is the following:
\(Kp = \frac{P(CO_{2}) P(CF_{4}) }{P(COF_{2} )^{2} } = 2.2 x 10^{6}\)
Since Kp>>>>1 ⇒ (P(CO₂) x P(CF₄)) > (P(COF₂))²
So, the partial pressures of the products (CO₂ and CF₄) are higher than the partial pressure of the reactant (COF₂).
Therefore, products will be favored at equilibrium at 298 K.
Write the balanced nuclear equation for the alpha decay of each isotope.
200/84Po
nuclear equation:
thorium-229
nuclear equation:
244/96Cm
nuclear equation:
bismuth-210
nuclear equation:
Answers
Explanation:
First, a quick revision of radioactive decay:
During alpha decay, an alpha particle is emitted from the nucleus —- it is the equivalent of a helium atom (i.e. it has a mass of 4 and an atomic number of 2). So, let's take the following question:
Polonium-210 is a radioisotope that decays by alpha-emission. Write a balanced nuclear equation for the alpha decay of polonium-210.
In symbols, the equation becomes
210/84Po--->?+4/2HE
The sums of the superscripts and of the subscripts must be the same on each side of the equation.
Take 4 away from the mass number (210-4 = 206)
Take 2 away from the atomic number (84-2 = 82). Lead is element number 82.
So, the equation is
210/84 Po--->206/82Pb+4/2He
Now let's try one for beta decay — remember that, in beta decay, a neutron turns into a proton and emits an electron from the nucleus (we call this a beta particle)
Write a balanced nuclear equation for the beta decay of cerium-144)
In nuclear equations, we write an electron as 0^-1e.
144/58Ce-->144/59Pr+^0-1e
Here's a fission reaction.
A nucleus of uranium-235 absorbs a neutron and splits in a chain reaction to form lanthanum-145, another product, and three neutrons. What is the other product?
We write a neutron as 1/0n, so the equation is
235/92U +1/0n--->145/57La+X+3 1/0n
Sum of superscripts on left = 236. Sum of superscripts on right = 148. So X
must have mass number = 236 – 148 = 88.
Sum of subscripts on left = 92. Sum of subscripts on right = 57. So X
must have atomic number = 92 – 57 = 35. Element 35 is bromine.
The nuclear equation is
235/92U+1/0n--->145/57La+88/35Br+31/0N
A nuclear decay leads to the formation of a product that has a different isotope from that of the parent.
What is a nuclear equation?A nuclear equation has to do with an equation in which one isotope is transformed into a different isotope.
For 200/84Po;
200/84Po ----> 4/2 He + 196/82Pb
For 244/96Cm
244/96Cm -----> 4/2 He + 240/94Pu
For bismuth-210
210/83Bi -----> 4/2 He + 206/81Tl
Learn more about nuclear decay:https://brainly.com/question/12224278
#SPJ2
The volume of a scuba tank is 10.0 L. It contains a mixture of nitrogen and oxygen at 290.0 atm.
What volume of this mixture could the tank supply to a diver at 2.40 atm?
Write out the answer
Answers
Answer:
each gas in the mixture add to give the total pressure (Ptot = P1 + P2 + P3 + ...) 1. ... After mixing, there will be the same number of moles of each gas, at the same T, just in a different volume. .OOL ... Pe=1697 atm P = 174_ atm. (1.000. ... Oxygen is pumped into a tank to supply breathing air for scuba diving, until the gas
Explanation:
The volume of the mixture will be "1208.33 L". A further solution is provided below.
The given values are:
Volume of scuba tank,
V₁ = 10 LPressure,
P₁ = 290 atmP₂ = 2.40 atmAccording to the Boyle's Law,
→ \(P_1V_1=P_2V_2\)
or,
→ \(V_2=\frac{P_1V_1}{P_2}\)
By putting the given values, we get
→ \(=\frac{290\times 10}{2.40}\)
→ \(= \frac{2900}{2.40}\)
→ \(= 1208.33 \ L\)
Thus the above answer is correct.
Learn more about volume of mixture here:
https://brainly.com/question/3841593
Question 9
Charles runs two laps around 500 m track. Waht is his total distance traveled?
distance traveled =
m
Answers
Answer:
1000 or 250
Explanation:
500x2=1000
or
500/2=250
17.3 L to mL conversiones sistema métrico
Answers
Answer:
17300ml
Explanation:
17.3 X 1000 = 17300ml
In fall, leaves may change from green to yellow or red. Explain in your own words what is happening inside the leaf with regard to plant pigments.
Answers
Answer:
The pigment that causes leaves to be green is chlorophyll. ... As chlorophyll goes away, other pigments start to show their colors. This is why leaves turn yellow or red in fall. In fall, plants break down and reabsorb chlorophyll, letting the colors of other pigments show through.
Which of the following represents the equation for volume?
L x H
L x W
L x W x H
W x H
Answers
Answer:
L× W×H
Please mark me as brainlist.Answer:
The correct answer is L x W x H :)
Based on the Law of Conservation of Mass,
what mass of products form when baking
soda decomposes?
NaHCO3 → Na₂CO3 + H₂O + CO₂
25.00 g
Give your answer to the correct number of
significant figures.
(g) Sodium Chloride
?g
Enter
Answers
Based on the Law of Conservation of Mass, the mass of products form when baking soda decomposes is 168 g/mole.
What is law of conservation of mass?Law of conservation of mass is defined as chemical reactions and physical changes cannot build or remove mass in an isolated system. The mass of the reactants and products in a chemical reaction must equal each other according to the law of conservation of mass.
\(\rm 2NaHCO_3 \rightarrow Na_2CO_3 + H_2O + CO_2\)
Mass of baking soda = 2 x molar mass
Molar mass of NaHCO₃ = 84.007 g/mole
Mass of baking soda = 2 x 84.007 g/mole
Mass of baking soda = 168.014 g/mole ≅ 168 g/mole
Thus, based on the Law of Conservation of Mass, the mass of products form when baking soda decomposes is 168 g/mole.
To learn more about Law of Conservation of Mass, refer to the link below:
https://brainly.com/question/28711001
#SPJ1
If you have 2 moles of gas at -57 degrees C in a 4-liter container, what is the pressure (in atm)
Answers
prop-1-yne + 2HBr/H2O2 = A;
A + 2H2O = B;
B + K2CO3(aq) = C;
C + heat = D;
D + HBr = E.
find the compounds A, B, C, D and E
Answers
Based on the given reactions, the compounds are as follows:
A: The specific product formed from the reaction between prop-1-yne and either 2HBr or H2O2.
B: The product formed when compound A reacts with 2H2O.
C: The product formed when compound B reacts with K2CO3(aq).
D: The product formed from the heat-induced reaction of compound C.
E: The product formed when compound D reacts with HBr.
Based on the given reactions, let's analyze the compounds involved:
Reaction 1: prop-1-yne + 2HBr/H2O2 = A
The reactant prop-1-yne reacts with either 2HBr or H2O2 to form compound A. The specific product formed will depend on the reaction conditions.
Reaction 2: A + 2H2O = B
Compound A reacts with 2H2O (water) to form compound B.
Reaction 3: B + K2CO3(aq) = C
Compound B reacts with K2CO3(aq) (potassium carbonate dissolved in water) to form compound C.
Reaction 4: C + heat = D
Compound C undergoes a heat-induced reaction to form compound D.
Reaction 5: D + HBr = E
Compound D reacts with HBr (hydrobromic acid) to form compound E.
For more such questions on compounds
https://brainly.com/question/704297
#SPJ8