What is SI unit and it's advantage
Answers
Related Questions
I will brainless who can answer this all pleaseeeeej

Answers
Answer:
cracks in the road, hot air balloons, tire bursts
Explanation:
cracks in the road: the road expand on heating
hot air balloons: Because the hot air inside the balloon bag increases in size faster than the container it stretches the bag so that it expands and displaces the colder (heavier) air outside the bag
tire bursts in hot days when filled full of air due to thermal expansion
PLEASE MARK BRAILIEST,THANKS <3
Consider the graph below. Which phase change is most likely taking place in this graph? a gas to a liquid a liquid to a solid a gas to a solid a solid to a liquid

Answers
Answer:
D) a solid to a liquid
Explanation:
EDGE 2022
which of the following is an indicator of a chemical reaction?
A. two different compounds mixing and remaining seperate.
B. changing states of matter (solid to liquid).
C. decreasing in size.
D. increasing in tempurature
Answers
Answer:
option . D
Increase in temperature
is an indicator of a chemical reaction
hope it helps
Answer:
answer is D
Explanation:
some signs of a chemical change are a change in colour and the formation of bubbles.
the five conditions of chemical change: colour change, formation of precipitate, formation of a gas ,odor change, temperature change.
I think it will use for you
Explain why iron from the blast furnace is harder than pure iron .
Answers
Iron produced in a blast furnace is harder than pure iron due to the presence of impurities and the way it is produced.
What happens in a blast furnace?In a blast furnace, iron ore is reduced to iron using coke as a reducing agent and a source of carbon. The high temperatures involved in this process result in the formation of a cast iron product that contains carbon and other impurities in the form of carbides and other compounds.
The presence of carbon and other impurities in the iron makes it harder and stronger than pure iron, which is relatively soft and ductile. The carbides formed during the reduction process act as tiny cutting edges, strengthening the iron and increasing its hardness.
This hardened iron is then melted and poured into molds to form pig iron, which can then be further processed to remove impurities and refine its properties. The end result is a stronger and harder iron product that is better suited for various applications, such as construction or manufacturing.
Learn more on blast furnace here: https://brainly.com/question/4259456
#SPJ1
initial velocity is used to study enzyme kinetics because a. the substrate concentration increases as the reaction proceeds. b. the substrate concentration decreases as the reaction proceeds. c. the presence of inhibitor slows down the reaction. d. the presence of inhibitor stops the reaction. e. cooperativity affects the reaction rate.
Answers
Initial velocity is used to study enzyme kinetics because b)the substrate concentration decreases as the reaction proceeds.
Initial velocity is used to study enzyme kinetics because it measures the rate of a reaction at the start of the reaction, prior to the reaction's completion. This allows researchers to measure the initial rate of reaction, which is influenced by a variety of factors, including substrate concentration, presence of inhibitors, and cooperativity.
Substrate concentration: When the substrate concentration increases, the reaction rate increases. When the substrate concentration decreases, the reaction rate decreases.Inhibitors: Inhibitors slow down the rate of reaction, and if the concentration of the inhibitor is high enough, the reaction can be completely stopped.
Cooperativity: Cooperativity affects the rate of reaction by creating a positive or negative feedback loop. In positive cooperativity, the rate of reaction increases as more enzyme molecules bind to the substrate, and in negative cooperativity, the rate of reaction decreases as more enzyme molecules bind to the substrate.
In conclusion, initial velocity is used to study enzyme kinetics because it measures the initial rate of reaction, which is affected by substrate concentration, presence of inhibitors, and cooperativity. The Correct answer is b).
Know more about Initial velocity here:
https://brainly.com/question/29110645
#SPJ11
What can the reader conclude about the effects of hurricanes?
They only cause damage near the equator.
They cause most of their damage when they reach land.
The damage is only bad if you are inside the eye of the storm.
People are safe from hurricanes if they stay inside of their homes.
Answers
RODINNIS
COURSES
onal Science
Attempt 1 of 2
Which of the following distinctions are used to identify sedimentary rock? Select all that apply.
o where is was formed
conditions it was formed under
n when it was formed
what it is composed of
how many layers it consists of
NEED HELP ASAP (check the picture)

Answers
where it was formed and. conditions it was formed under
For the reaction magnesium plus hyrdogen chloride yeilds hydrogen and magnesium chloride, calculate the percent yield of magnesium chloride if 100. G of magnesium react with excess hydrochloric acid to yield 330. G of magnesium chloride
Answers
Answer:
Percentage yield of MgCl₂ = 83.4%
Explanation:
We'll begin by writing the balanced equation for the reaction. This is illustrated below:
Mg + 2HCl —> MgCl₂ + H₂
Next we shall determine the mass of of Mg that reacted and the mass of MgCl₂ produced from the balanced equation. This can be obtained as follow:
Molar mass of Mg = 24 g/mol
Mass of Mg from the balanced equation = 1 × 24 = 24 g
Molar mass of MgCl₂ = 24 + (35.5×2)
= 24 + 71
= 95 g/mol
Mass of MgCl₂ from the balanced equation = 1 × 95 = 95 g
SUMMARY:
From the balanced equation above,
24 g of Mg reacted to produce 95 g of MgCl₂.
Next, we shall determine the theoretical yield of MgCl₂. This can be obtained as follow:
From the balanced equation above,
24 g of Mg reacted to produce 95 g of MgCl₂.
Therefore, 100 g of Mg will react to produce = (100 × 95)/24 = 395.83 g of MgCl₂.
Thus, the theoretical yield of MgCl₂ is 395.83 g
Finally, we shall determine the percentage yield of MgCl₂. This can be obtained as follow:
Actual yield of MgCl₂ = 330 g
Theoretical yield of MgCl₂ = 395.83 g
Percentage yield of MgCl₂ =?
Percentage yield = Actual yield /Theoretical yield × 100
Percentage yield = 330 / 395.83 × 100
Percentage yield of MgCl₂ = 83.4%
Which combustion reaction will produce more energy, ethanol C2H5OH or propane C3H8,? Use evidence from your calculations
to support your answer.
Answers
Propane produces more energy as compared to ethanol during burning.
How much energy is produced by ethanol and propane?Energy produce by ethanol is 10.45 kilojoules per gram whereas propane release 46 kilojoules per gram of energy when burn so by comparing these two chemicals we can conclude that propane produces more energy as compared to ethanol during burning.
Learn more about ethanol here: https://brainly.com/question/281073
why is the water a liquid and h2s a gas ?
Answers
Explanation:
This is because the hydrogen bonding in water H2O is stronger than that is hydrogen sulfide H2S.
85 g of AgNO3 represents ___?
moles of AgNO3.
a) 0.50
b) 0.60
c) 1.5
d) 2.0
Answers
Answer:
a
n=m/M
mass if 85 gms and molar mass is 169.87 g/mol
85/169.87=0.50038 moles
Explanation:
85 g of AgNO3 represents 0.50 moles of AgNO3. Hence option a is correct.
What are moles?Moles are defined as the mass of a system that has the same number of constituent components as there are atoms in 0.012 kg of carbon 12. In chemistry, a mole is a common unit of measurement for huge concentrations of very small objects like atoms, molecules, or other predetermined particles. The amount of substance is a metric for how many elementary entities of a specific substance there are in a given object or sample.
The number of moles cam be calculated as molar mass multiply by molecular mass.
n=m/M
Molar mass is 169.87 g/mole
Mass is 85 grams
so, n = 85/169.87
n = 0.50038 moles
Thus, 85 g of AgNO3 represents 0.50 moles of AgNO3. Hence option a is correct.
To learn more about moles, refer to the link below:
https://brainly.com/question/26416088
#SPJ2
Calculate the densities of the objects using the volumes found by displacement. Record your data
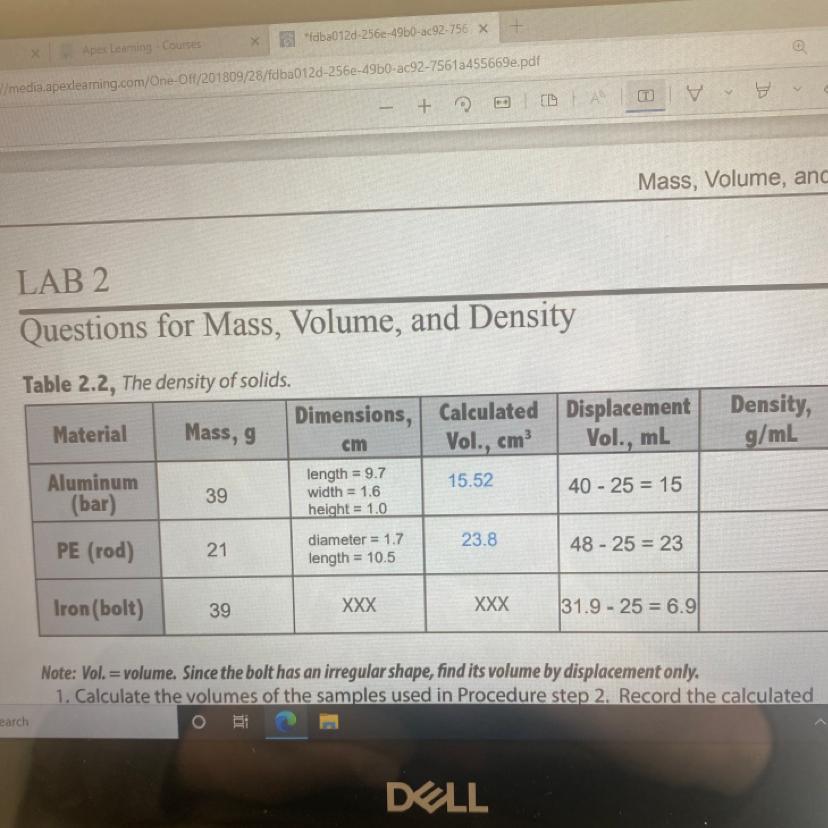
Answers
The densities of the substances are;
Aluminum bar = 2.6 g/ml
PE rod = 0.91 g/mL
Iron bolt = 5.65 g/mL
What is density?The term density is defined as the ratio of the mass to the volume of the object. We know that mass is an intrinsic property. This means that the density of the object can be used to identify what the object that is under study is. Let us now try to find the density of each of the objects.
In this case, we have the mass and the volumes of the objects as they have been shown in the table that have here. We can now be able to find the volume of each of the objects.
Density of aluminum bar = 39 g/ 15 g/mL = 2.6 g/ml
Density of the PE rod = 21 g/23 mL = 0.91 g/mL
Density of iron bolt = 39 g/6.9 mL = 5.65 g/mL
Learn more about density:https://brainly.com/question/15164682
#SPJ1
An atom X that has 12 protons and 12 electrons, loses 2 electrons to form an ions, What will be the ion? What will be the number of protons and electrons in the ions?
Answers
An atom X that has 12 protons and 12 electrons, loses 2 electrons to form an ions, the charge of ion will be X⁺² the number of protons 12 and electrons in the ions is 10.
An atom X has 12 protons and 12 electrons that means X is a neutral atom and the atomic no. is 12 because total no. of protons is equal to atomic number. When it looses the 2 electrons then atom becomes positively charged ion . the charge on the ion is +2.
now, the no. of protons will be = 12
number of electrons will be = 12 - 2 = 10
Thus, An atom X that has 12 protons and 12 electrons, loses 2 electrons to form an ions, the charge of ion will be X⁺² the number of protons 12 and electrons in the ions is 10.
To learn more about Atom here
https://brainly.com/question/1566330
#SPJ1
describe how to identify the smell of gas in the laboratory
Answers
Answer:
When you are in the laboratory and take a direct sniff the chemicals you are using, you run the risk of damaging your mucous membranes or your lungs. When its necessary to smell chemicals in the lab, the proper technique is to cup your hand above the container and waft the air towards your face.
Gas is a naturally odourless substance, but the completely harmless artificial smell is added to make it more detectable. The substance is called mercaptan and gives off a strong sulphur like smell.
One step in making para-aminobenzoic acid, PABA, an ingredient in some sunscreens, involves replacing one of the hydrogen atoms in a toluene molecule (C7H8) with an NO2 group. Water is also formed. Calculate how many molecules of the nitrotoluene product you can make if you start with 550g of toluene and plenty of nitric acid.
Answers
To calculate the number of molecules of the nitrotoluene product that can be formed, we need to convert the given mass of toluene (550g) into the number of moles and then use the stoichiometry of the reaction to determine the number of moles of the product. Here's the step-by-step calculation:
Calculate the number of moles of toluene:
Molar mass of toluene (C7H8) = 92.14 g/mol
Number of moles of toluene = Mass of toluene / Molar mass of toluene
Number of moles of toluene = 550 g / 92.14 g/mol ≈ 5.98 mol
Use the stoichiometry of the reaction to determine the number of moles of the product:
The balanced chemical equation for the reaction is:
C7H8 + HNO3 → C7H7NO2 + H2O
From the balanced equation, we can see that 1 mole of toluene reacts with 1 mole of nitric acid to produce 1 mole of nitrotoluene.
Therefore, the number of moles of nitrotoluene = Number of moles of toluene = 5.98 mol
Convert the number of moles of nitrotoluene to molecules:
Avogadro's number states that 1 mole of any substance contains 6.022 x 10^23 molecules.
Number of molecules of nitrotoluene = Number of moles of nitrotoluene x Avogadro's number
Number of molecules of nitrotoluene = 5.98 mol x (6.022 x 10^23 molecules/mol) ≈ 3.60 x 10^24 molecules
Therefore, starting with 550g of toluene and plenty of nitric acid, you can produce approximately 3.60 x 10^24 molecules of the nitrotoluene product.
To know more about nitrotoluene click this link-
https://brainly.com/question/30883344
#SPJ11
(20 points and excuse any offensive or seemingly bad language in this question) Budding and regeneration are two types of asexual reproduction.
Answers
Answer: true
Asexual reproduction is the process by which an organism is produced from a single parent cell. There are four major forms of asexual reproduction - budding, binary fission, regeneration and parthenogenesis. ... Regeneration is a type of asexual reproduction in which the organism is capable of regrowing certain body parts.
Explanation:
id k what u r talking abt but hope this helps
The chemical formula for naphthalene is C10H8. It’s used to make mothballs and pesticides.
In 4C10H8, the coefficient is , the subscript of carbon is , and the subscript of hydrogen is .
Answers
In \(4C_{10}H_8\), the coefficient is 4, the subscript of carbon is 10, and the subscript of hydrogen is 8.
Coefficients of chemical formulasChemical formulas can be empirical or molecular. Empirical formulas have the lowest possible whole-number ratio of atoms that make up substances. Molecular formulas, on the other hand, could have whole-number ratios that could be in multiples.
Thus, each atom in chemical formulas has its respective subscripts which indicate the amount of the atom present in the formula. In chemical equations, the coefficients are the number written before chemical formulas.
Thus, in \(4C_{10}H_8\), the coefficient is 4, the subscript of carbon is 10, and the subscript of hydrogen is 8.
More on chemical formulas can be found here: https://brainly.com/question/29031056
#SPJ1
Polyelectrolytes are typically used to separate oil and water in industrial applications. The separation process is dependent on controlling the pH. Fifteen (15) pH readings of wastewater following these processes were recorded. Is it reasonable to model these data using a normal distribution? 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 10.0 10.5 7.6 11.4 11.4 10.0 Yes, it passes the "fat pencil" test. Therefore, a normal distribution is a reasonable model. No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O Yes, it passes the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O No, it does not pass the "fat pencil" test. Therefore, a normal distribution is a reasonable model.
Answers
No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. Option B is the correct answer.
The "fat pencil" test is a quick visual check to determine if a dataset can be reasonably approximated by a normal distribution. In this case, the pH readings of wastewater show a significant deviation from a normal distribution. The presence of several low pH values (1.0) and a few high pH values (10.0, 10.5, 11.4) indicate a non-normal distribution with skewness and potential outliers. Therefore, it is not reasonable to model these data using a normal distribution.
Option B is the correct answer.
You can learn more about normal distribution at
https://brainly.com/question/4079902
#SPJ11
A balloon contains 25.0 L of helium gas at 177 kPa. What is the volume when the balloon rises to an altitude where the pressure is only 43.0 kPa ? Assume the temperature remains constant.
A.34.2 L
B.68.8 L
C.46.5 L
D.103 L
Answers
this had me thinking
what is smaller than an atom... is there really anything smaller than an atom??!! i dont think there is is there?
Answers
Answer:
the protons, neutrons, and electrons that make up the atom?
Explanation:
Please helppppppppppppppppppp
50 ppoints

Answers
Answer:
I can’t see the whole page
Explanation:
What kind of intermolecular forces act between a hydrogen molecule and an argon atom? a. Dipole-dipole interaction
b. Interaction c. Hydrogen-bonding d. Dispersion forces e. lon-dipole interaction
Answers
The kind of intermolecular forces that act between a hydrogen molecule and an argon atom is dispersion forces (option D).
Intermolecular forces are the attractive forces that act between molecules or atoms. The strength of these forces determines the physical properties of substances such as boiling points, melting points, and solubility. The five main types of intermolecular forces are: Dispersion forcesDipole-dipole interactionsHydrogen bondingIon-dipole interactionsIn the given case, a hydrogen molecule is a nonpolar molecule consisting of two hydrogen atoms sharing electrons equally between them. An argon atom is also a nonpolar atom because it has a complete octet of electrons and no permanent dipole moment.
Therefore, the only intermolecular force that can act between them is the dispersion force. Dispersion forces are caused by temporary dipoles that are induced in nonpolar molecules or atoms when they come close to each other. These temporary dipoles cause the electron distribution in adjacent molecules or atoms to become distorted, leading to a weak attraction between them. Since hydrogen molecules and argon atoms are both nonpolar, they experience dispersion forces when they come close to each other. Hence, option D is the correct answer.
Learn more about intermolecular forces at brainly.com/question/9007693
#SPJ11
Which ions produce similar colors in the flame tests?
Answers
Answer:
Two ions that produced similar colors in the flame test were Ca+2 and Sr+2. 3.
Explanation:
The colors are produced when an electron jumps to a higher level and then jump back down.
Ba2+ and Cu2+ and Sr2+ and Li+ were the pair with the similar color. Sr and Li displayed red colors, while Ba and Cu had mild greenish yellowish hues.
Why do some ions in the flame test generate colors that are similar?
The precise sizes of the potential energy jumps differ from metal to metal. As a result, the flame color of each metal will differ due to its unique spectral line pattern. The movement of the electrons in the metal ions contained in the compounds results in the hues of the flame.
The energy released by each electron when it returns to its initial condition determines the hue of the light that is produced.
To learn more about ions in the flame refer to:
https://brainly.com/question/28715571
#SPJ2
Which particles have approximately the same size and mass as each other?
A Neutrons and Electrons
B Protons and Neutrons
C Electrons and Protons
D None of these
Answers
Answer:
B.
Explanation:
Thus we can see that the mass of protons and mass of neutrons are approximately equal.
5. Which of these elements has the greatest atomic radius? *
Be
Mg
Ra
Ba
Answers
Atomic number for Be is 4
Atomic number for Mg 12
Atomic number for Ra 88
Atomic number for Ba 56
g a reaction which is exothermic and has an overall increase in entropy is a) spontaneous only at high t b) spontaneous only at low t c) always spontaneous d) spontaneous in the reverse direction.
Answers
A reaction which is exothermic and has an overall increase in entropy is
A) spontaneous only at high T
B) spontaneous only at low T
C) always spontaneous
D) spontaneous in the reverse direction.
The correct option is C) i.e., always spontaneous
Spontaneity is determined by the free energy. When ΔGΔG is negative, it is spontaneous.
ΔG=ΔH−TΔSΔG=ΔH−TΔS
The problem indicates that the ΔSΔS is positive. If the reaction is exothermic, this means that the ΔHΔH is negative.
ΔGΔG is going to be negative no matter the temperature.
A spontaneous process is one that occurs on its own, without any energy input from the outside. For example, a ball will roll down an incline; water will flow downhill; ice will melt into water; radioisotopes will decay, and the iron will rust.
To learn more about spontaneous reactions visit:
https://brainly.com/question/13790391
#SPJ4
brainly which process forms all elements up to and including iron, except light elements such as hydrogen and helium?
Answers
Nuclear fusion is the process which forms all elements up to and including iron, except light elements such as hydrogen and helium.
Nuclear Fusion reactions power the solar and different stars. In a fusion response, two mild nuclei merge to shape a single heavier nucleus.
The method releases power due to the fact the total mass of the ensuing single nucleus is less than the mass of the 2 unique nuclei. The leftover mass turns into electricity.
For years, the science has proved difficult to master. However over the last year, nuclear fusion has inched in the direction of fact. Scientists are mere years from getting extra energy out of fusion reactions than the power required to create them, they stated.
Learn more about nuclear fusion here:- https://brainly.com/question/17870368
#SPJ4
a substance that does not take shape of a container
Answers
Answer: gasses
Explanation: Solids have a definite shape and volume. Liquids have a definite volume, but take the shape of the container. Gases have no definite shape or volume.
The density of aluminum is 2.7 g/ml. What is the volume of 8.1 g?
Answers
Answer:
volume= 3
Explanation:
D=m/v
2.7=8.1/v
2.7v=8.1
8.1/2.7= 3
8.1/3= 2.7 which is the density!
Answer:
3 mL
Explanation:
\(V = \frac{8.1 g}{3 mL} = 2.7 g/mL\)
An atom has an atomic mass of 223 and an atomic number of 87. Calculate the number of neutrons.
Answers
Answer:
136 neutrons
Explanation:
To find the number of neutrons of an atom, you subtract the atomic number (number of protons) from the atomic mass.
223 - 87 = 136