what is Brownian motion
Answers
Answer:
This refers to the random and erratic movement of microscopic particles that are suspended in any fluid, like water or oil. Brownian motion is the result of the impact of the random bombardment of microscopic particles by the variety of fast-moving molecules that constitute the fluid.
Explanation:
Answer:
the erratic random movement of microscopic particles in a fluid as a result and continuous bombardment from molecules of the surrounding medium
Related Questions
Solid to Gas
As a change is state takes place, heat _______ and temperature _______.
A. Increases, stay the same
B. Stay the same, changes
C. Increases, increases
Answers
Answer:
c. c. c c c c c cçcc. ccccccccc
Explanation:
ccc cc
When solid converts to gas then heat is required to break the intermolecular forces of attraction between the particles. In this process heat increase but temperature remains the same. So option A is correct option.
What is phase transition?Phase transition is a process in which transition takes place from one state to another of a medium on changing temperature or pressure. Phase transition is a physical process as there is no breaking of old bond and forming of new bonds takes place.
During phase transition temperature remain constant as the extra heat that is given to the system that goes into breaking of intermolecular forces of attraction between the particles. So overall temperature remains same but heat keeps on increasing. Hence option A is correct.
To learn more about phase transition, here:
https://brainly.com/question/3255181
#SPJ2
If you have a graduated cylinder containing 15.50 mL of water and this volume changes to 17.97 mL after a metal with a mass of 17.95 g is dropped into the graduated cylinder, then what is the density of this metal?
Answers
Answer:
Explanation:
density = mass / volume
mass = 17.95 grams
volume = 17.97 - 15.50 mL = 2.45 mL = 2.45 cc
density = 17.95 /(2.45) = 7.327 grams / cc
to the best of your knowledge, classify each of the following as an element, compound, or mixture. explain how your everyday experiences influenced your response. a. silver coin b. air c. coffee d. soil
Answers
a. Silver coin - Element (Silver is a pure element and is not chemically combined with any other element in a silver coin)
b. Air - Mixture (Air is a mixture of gases, primarily nitrogen and oxygen, with trace amounts of other gases and particles)
c. Coffee - Mixture (Coffee is a mixture of various compounds such as water, caffeine, and organic compounds that give it its flavour and aroma)
d. Soil - Mixture (Soil is a mixture of various substances such as minerals, organic matter, water, and air)
My everyday experiences influenced my response because I have come across these examples in my daily life and have been taught about them in science classes. For example, I know that air is composed of different gases and particles, and that soil is made up of a mixture of substances, including minerals and organic matter. Similarly, I know that a silver coin is made of pure silver and that coffee is made of various compounds.
To learn more about Compounds click here:
https://brainly.com/question/14658388
#SPJ4
The table shows the relative atomic masses of some common elements. Use this information to work out the relative formula mass of magnesium sulfate. The formula for magnesium sulfate is MgSO4.
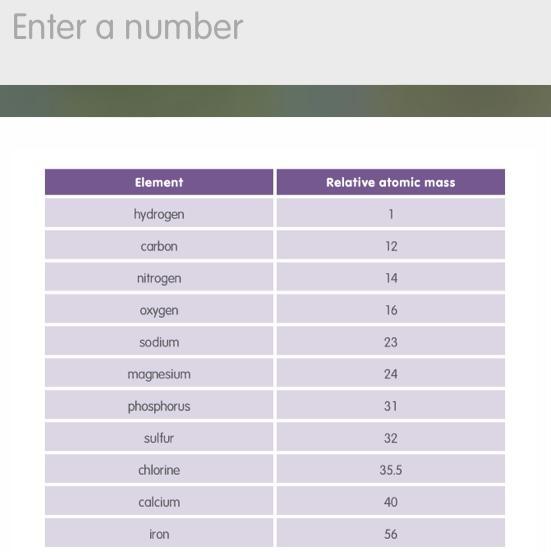
Answers
The relative atomic mass of Mg is 24.3, the relative atomic mass of S is 32.1, and the relative atomic mass of O is 16.0.
What is relative atomic mass?The relative atomic mass is a unit of measurement used in chemistry to express the mass of an atom of an element.It is defined as the ratio of the mass of an atom of an element to one twelfth of the mass of an atom of carbon-12.It is often used to compare the masses of atoms of different elements.The relative atomic mass is usually represented by the symbol "A" or "Ar" and is measured in atomic mass units (amu).It is important to note that the relative atomic mass is different from the atomic mass, which is the actual mass of an atom in grams.To calculate the relative formula mass of magnesium sulfate, we add up the relative atomic masses of each element in the formula:Mg = 24.3S = 32.14 x O = 4 x 16 = 64.0So, the relative formula mass of magnesium sulfate is 24.3 + 32.1 + 64.0 = 120.4.To learn more about relative atomic mass refer:
brainly.com/question/28882057
#SPJ1
Iron reacts with oxygen to form rust according to the equation: 2 Fe + 3 O2 --> 2 Fe2O3
If you react 60,5 moles of oxygen gas, how many moles of rust can you form?
Answers
Answer: 40.3
Explanation:
In the reaction, we see that for every 3 moles of oxygen gas consumed, 2 moles of rust are formed.
So this means that if 60.5 moles of oxygen gas are consumed, then (60.5/3)(2)=40.3 moles of rust can be formed.
Which statements are true about light waves? (Select all that apply.)
Light waves are sun waves.
Light waves are frequency waves.
Light waves are electromagnetic waves.
Light waves are transverse waves.
PLEASE SELECT MORE THAN ONE
WILL MARK BRAINLY :D✌️
Answers
Answer:
only transverse and electromagnetic waves
Explanation:
Light waves are electromagnetic waves.Electromagnetic waves or EM waves exist waves that are created as a consequence of vibrations between an electric field and a magnetic field.
What are electromagnetic waves?In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, propagating via space, and having electromagnetic radiant energy. It contains radio waves, microwaves, infrared, (visible) light, ultraviolet, X-rays, and gamma rays. All of these waves form parts of the electromagnetic spectrum.
Light waves are electromagnetic waves.Electromagnetic waves or EM waves exist waves that are created as a consequence of vibrations between an electric field and a magnetic field.
Learn more about electromagnetic waves refer to:
https://brainly.com/question/26960785
#SPJ2
how long does it take for stirring and heat to dissolve in water?
Answers
Answer:
5-20 mins
Explanation:
Help needed ASAP, I will mark your answer as brainliest.

Answers
Visit the interactive periodic table and locate the element neon (Ne). Use the information within the square to answer these questions.

Answers
Answer:
What is the atomic number of neon?
10
What is the atomic mass of neon? Give your answer to the nearest tenth.
20.2
The periodic table is a representation of the periodic elements based on atomic numbers. The atomic number of neon is 10, and the atomic mass is 20.2 amu.
What are the properties of neon?Neon is categorized as a stable gas that belongs to the noble gas group. It has been represented as Ne and is a monoatomic gas with no odor or color. It has an atomic number of 10 and an electronic configuration as, [He] 2s²2p⁶.
The atomic mass of neon elements is 20.2 amu and is said to have full electrons per shell. They do not need to donate or accept any electron as they have a balanced orbital. They have the property of fluorescence and are used in electric signs and lamps.
Therefore, neon is placed in group 18 and has atomic number 10 and a mass of 20.2 amu.
Learn more about neon, here:
https://brainly.com/question/8226528
#SPJ6
Finish the essay technology improves nursing
Technology has helped correct medical errors with safety checks, finding an early diagnosis of a disease, and more. Technology is a huge part of nursing........
Answers
Answer: come on man its not that hard
Explanation:
A particular tank of oxygen gas contains 785 L at 21°C.If the pressure remains constant,what volume will the gas occupy if the temperature is changed to 28°C? (Write procedure)
Answers

anyone know this? i need help

Answers
-1708 + - 2325 = -4033 J
Products:
-4548 +-59.1+726 = - 3881.1 J
Products - Reactants = -3881.1 - - 4033
= +151.9 J
(Reaction is endothermic)
Hope this helps :))
The pka of acetate is 4.76. what is the ph of a solution made by combining 150 ml of 1.1 m acetic acid and 175 ml of 0.6 m sodium acetate?
Answers
The pH of the solution is approximately 4.62.
The pKa of acetate is 4.76. To find the pH of the solution, we need to calculate the concentrations of acetic acid and sodium acetate, and then use the Henderson-Hasselbalch equation.
First, we calculate the moles of acetic acid (0.150 L x 1.1 M = 0.165 moles) and sodium acetate (0.175 L x 0.6 M = 0.105 moles).
Next, we calculate the concentrations of acetic acid and acetate ions (0.165 moles / 0.325 L = 0.508 M and 0.105 moles / 0.325 L = 0.323 M, respectively).
Now, we can plug these values into the Henderson-Hasselbalch equation:
pH = pKa + log10([acetate]/[acetic acid]) = 4.76 + log10(0.323/0.508) ≈ 4.76 - 0.14 = 4.62.
Therefore, the pH of the solution is approximately 4.62.
Learn more about concentrations here:
https://brainly.com/question/30862855
#SPJ11
Drag each label to the correct location.
We use specific words to describe each type of change in state. Label the arrows to describe the changes of state they
represent
gas
liquid
solid
melting freezing condensation
evaporation sublimation
deposition

Answers
solid —> liquid = melting
liquid —> solid = freezing
solid —> gas = sublimation
gas —> liquid = condensation
gas —> solid = deposition
What is the total amount of matter in an ecosystem doing?
Answers
AnsThe flow of matter in an ecosystem is not like energy flow. Matter enters an ecosystem at any level and leaves at any level. So, its always flowingwer:
Explanation:
If we mixed 15 grams of water with 10 grams of sugar we can expect the mass of the sugar water to be what? Write
the mass and defend your answer using a scientific principle.
Answers
Answer:
25 grams
Explanation:
The law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant, as system's mass cannot change.
Which of the following statements is correct?
Group of answer choices
Water and nitrogen are both compounds.
Water and nitrogen are both elements.
Water is an element and nitrogen is a compound.
Water is a compound and nitrogen is an element.
Answers
Answer:
water is a compound and nitrogen is an element
Explanation:
water is a compound and also a molecule because it is a combination of two different element, while nitrogen is an atom or an element
Answer: Water is a compound and nitrogen is an element.
Explanation:
Water = H20 = 2 Hydrogen atoms and 1 Oxygen atom
Nitrogen (N) element of Group 15 of the periodic table
Identify three sources of point-source pollution.
Answers
methanol is produced using anaerobic digestion of acetic acid. if 0.3 g of co2 is produced per g acetic acid consumed, calculate the yield of methanol from the substrate (g methanol/g acetic acid).
Answers
The yield of methane from acetic acid is 0.129 g CH4/g CH3COOH.
Methanol is produced using anaerobic digestion of acetic acid. If 0.3 g of CO2 is produced per g acetic acid consumed, the yield of methanol from the substrate (g methanol/g acetic acid) can be calculated as follows:
Solution:
Balanced reaction for anaerobic digestion of acetic acid is:
CH3COOH → CH3COO- + H+ H+ + e- → ½ H2
Hence,CH3COOH → CH3COO- + H+ + ½ H2 For every acetic acid molecule utilized, one methane (CH4) molecule and one carbon dioxide (CO2) molecule are produced.
CH3COOH → CH4 + CO2If 0.3 g of CO2 is produced per g acetic acid consumed, then the yield of methane (CH4) from acetic acid can be calculated as follows:
Yield of methane from acetic acid = (mass of methane produced) / (mass of acetic acid consumed)For each mole of methane produced, one mole of acetic acid is consumed.
So, the molar mass of acetic acid and methane is used to convert between mass and moles.
Yield of methane from acetic acid = (moles of methane produced x molar mass of methane) / (moles of acetic acid consumed x molar mass of acetic acid)
= (0.3 g CO2 / 44.01 g CO2/mol) x (16.04 g CH4/mol) / (1 g CH3COOH / 60.05 g CH3COOH/mol)
= 0.129 g CH4/g CH3COOH
To know more about anaerobic digestion visit
https://brainly.com/question/28712855
#SPJ11
While performing this experiment, we ignored the effect of the concentration of the bleach on the rate of the reaction in all four trials. Why were we able to do so? Select all responses that apply. A) The presence of the bleach in the reaction mixture is overshadowed by the presence of the dye and so the bleach does not affect the reaction rates B) Bleach is known to be zero order for this reaction C) We only care about the effect of the Allura Red dye on the rate of the reaction and so having excess bleach ensures the concentration of the bleach remains constant in the reaction D) Because the bleach was in excess, the order of the reaction with respect to the bleach is pseudo-zero
Answers
C) Because we only care about the effect of the Allura Red dye on the rate of the reaction, having excess bleach ensures that the concentration of the bleach in the reaction remains constant.
D) Because the bleach was in surplus, the reaction order is pseudo-zero with regard to the bleach.
What does order of a reaction signify?The order of a chemical reaction is a measure of how the reaction rate varies with the concentration of the reactants. It describes the relationship between the rate of the reaction and the concentrations of the reactants, and is usually determined experimentally.
The order of a chemical reaction is an assessment of how the reaction rate varies with the concentration of the reactants. The rate law equation can be expressed in the form:
rate = \(\mathrm k[A]^x [B]^y [C]^z\)
where k is the rate constant, [A], [B], and [C] are the concentrations of the reactants, and x, y, and z are the orders of the reaction with respect to A, B, and C, respectively. The sum of the orders with respect to all reactants is called the overall order of the reaction.
These two options correctly explain why the effect of the concentration of the bleach was ignored in the experiment. Option A is not correct because the presence of bleach is a necessary component of the reaction and is not overshadowed by the dye. Option B is not correct because the order of the reaction with respect to bleach is not given and would need to be determined experimentally.
To know more about reactants, visit:
brainly.com/question/17096236
#SPJ1
A pure gold ring weighs 23.5 grams. How many atoms of gold
are in the ring?
Answers
The number of atoms of gold in the pure ring are 7.18 × 10²² atoms.
HOW TO CALCULATE NUMBER OF ATOMS?The number of atoms in a substance can be calculated by multiplying the number of moles of the substance by Avogadro's number.
The number of moles in the gold (Au) can be calculated by dividing the mass of gold by its molar mass (196.97g/mol).
no. of moles = 23.5g ÷ 196.97g/mol
no. of moles = 0.119mol
Number of atoms in Au = 0.119 × 6.02 × 10²³
no. of atoms = 7.18 × 10²² atoms.
Therefore, the number of atoms of gold in the pure ring are 7.18 × 10²² atoms.
Learn more about number of atoms at: https://brainly.com/question/15959704
What large city in Texas will have its third day of rain?
Answers
Answer:
houston, texas
Explanation:
i hope this helps :)
how can splashes from the magnetic stirring of the titration be avoided?
Answers
It is possible to reduce splashing during magnetic stirring and minimize the risk of errors or contamination during titration if adequate steps are taken.
What causes splashes during titration?Splashes during titration can be caused by the magnetic stirring bar, which can cause drops of the titrant or sample to fly out of the container.
Here are some tips to avoid splashes during magnetic stirring:
Use an appropriate stirring speed: When the stirring speed is too high, it can create more turbulence and cause splashing. Using a moderate speed that is enough to keep the solution mixed but not too fast can help reduce splashing.
Use a larger container: A larger container can help reduce splashing by providing more space for the solution to move around. This reduces the chances of drops hitting the sides of the container and splashing out.
Use a splash guard: A splash guard can be placed over the container to prevent the drops from flying out. A simple splash guard can be made using a piece of paper or plastic film with a hole in the center for the stirring rod.
Adjust the position of the stirring bar: The position of the stirring bar can also affect the amount of splashing. It should be placed in the center of the container, and the depth should be adjusted so that it is not too close to the surface of the solution.
Use a lower flow rate: If the titrant is being added to the sample through a burette, a lower flow rate can help reduce splashing. This allows the drops to be smaller and less likely to fly out of the container.
Learn more about magnetic stirring here: https://brainly.com/question/13339404
#SPJ1
If 1.0 mole of C8H18 are available to completely react
with enough O2, how many moles of H20 can be produced?
Answers
Answer: 1 mol C8H18 produces 9 mol H2O
Explanation: reaction : C8H18 + 12.5 O2 ⇒ 8 CO2 + 9 H2O
Frozen red cells that have been prepared with high glycerol methods (40% glycerol) can be stored up to 10 years if held at which of the following temperatures?
- 65o C or lower
- 20o C or lower
- 10o C or lower
- 0o C or lower
Answers
Storing red blood cells (RBCs) requires proper preservation techniques to maintain their viability and functionality. One method is the high glycerol preservation method, which uses a high concentration of glycerol (40%) to protect the cells during storage. The temperature at which the RBCs are stored also plays a crucial role in determining their longevity.
Out of the options listed, the correct temperature for storing RBCs prepared with high glycerol preservation method is at 0°C or lower. At this temperature, the glycerol solution slows down cellular metabolism, preserving the viability and functionality of the RBCs for a longer period of time. Storing RBCs at 65°C or lower may damage the cells, rendering them unusable for transfusion. Similarly, storage at 20°C or lower may result in short-term preservation but is not ideal for long-term storage as it may cause a significant reduction in RBC viability. Storing RBCs at 10°C or lower may provide short-term preservation, but it may not be optimal for long-term storage as the low temperature may cause freezing of the cells, leading to irreversible damage.
Here you can learn more about red blood cells (RBCs)
https://brainly.com/question/4414904#
#SPJ11
Which of the following is a valid conversion factor? (3 points)
1 cm3/1L
1 hm/1,000m
1,000cg/1g
1,000mL/1L
Answers
Your last option
A pipet is used to measure out 10 ml of water. if the mass of this volume of water is 9.990 g and the density of water is given as 0.9978 g/ml. What is the actual volume of water measured out?
Answers
The actual volume of water measured out 10.012 mL.
We know that every three-dimensional object occupies some space. Volume is defined as the space that is measured in terms of its volume. Volume of any substance changes according to the mass.
Mass of water taken = 9.990 gram,
Density of water taken = 0.9978 gram/ml
We know formula volume in mL = mass in gram / density in gram per mL
Substitute values in this formula to find volume of water taken in mL
Volume of water taken in mL = 9.990/0.9978 = 10.012 ml
Hence, actual volume of water measured is 10.012 ml.
Learn more about volume from the link given below.
https://brainly.com/question/11090150
#SPJ4
as the temperature of a gas decreases is volume
Answers
Answer:
it's volume also decrease
Values for the molar mass of hydrogen, oxygen, and water molecules are
given in the table below. What mass of water is formed when 2 moles of
hydrogen react with 1 mole of oxygen to form water?
Molecule
Molar mass (g/mol)
H2
2.02
02
32.00
H20
18.01
A.9.00 g
B. 36.02 g
C. 2.00 g
D. 18.01 g
Answers
Answer:
36.02g bbbbbbbbbbb hbbnjkkkj
Answer:
36.02g
Explanation:
Hope this helps!
Which equation represents a spontaneous reaction?
A) Ca + Ba2+ to Ca2+ + Ba
B) Co + Zn2+ to Co2+ + Zn
C) Fe + Mg2+ to Fe2+ + Mg
D) Mn + Ni2+ to Mn2+ + Ni
Answers
Answer:Mn+Ni2+-->Mn2+ + Ni
Explanation:
Castle Learning said it
How do you determine if the reaction is spontaneous?
If ΔH is negative, and –TΔS positive, the reaction will be spontaneous at low temperatures (decreasing the magnitude of the entropy term).
What is the meaning of spontaneous reaction?
When both of these conditions are met, the reaction occurs naturally. A spontaneous reaction is a reaction that favors the formation of products at the conditions under which the reaction is occurring.
Learn more about spontaneous reaction here : brainly.com/question/24376583
#SPJ2