what is a renewable resource ?
Answers
Answer:
A renewable resource is a resource that can be made, Ex: trees or energy.
Explanation:
Answer:
energy source that cannot be depleted.
Explanation:
Related Questions
How does a catalyst increase reaction rate?
By increasing the activation energy
By increasing the amount of reactant
By increasing the contact between particles
By increasing the space between particles
Answers
you need to prepare 100ml of a ph=4 buffer solution using:______.
Answers
To prepare a 100 mL buffer solution with a pH of 4, you can use a combination of a weak acid and its conjugate base. In this case, a suitable option is the acetic acid (CH3COOH) and sodium acetate (CH3COONa) buffer system.
To prepare the buffer solution, you need to calculate the appropriate amounts of acetic acid and sodium acetate to achieve the desired pH. The pH of a buffer solution is determined by the pKa value of the weak acid. In the case of acetic acid, the pKa is approximately 4.76. Using the Henderson-Hasselbalch equation, pH = pKa + log([A-]/[HA]), you can determine the ratio of the conjugate base (A-) to the weak acid (HA) required to achieve the desired pH. Once you have the ratio, you can calculate the specific amounts of acetic acid and sodium acetate needed based on their concentrations to prepare a 100 mL buffer solution. The buffer solution will maintain its pH value even with the addition of small amounts of acid or base, making it suitable for pH regulation in various applications.
To learn more about conjugate base click here,
brainly.com/question/30086613
#SPJ11
Explain why the process of mining uranium involved in the use of nuclear energy has the greatest environmental impact of the entire process.
Answers
Uranium is a radioactive substance, which is extremely reactive. As a consequence, it cannot be found in the environment in its elemental state. During uranium, mining disturbances can influence both the quality and quantity of the surface water.
Several of the effects of uranium mining are identical to those experienced in other kinds of mining.
Due to the harmful effects of radioactive substances, the uranium mining for the use of nuclear energy has the greatest environmental influence.
a tghin layer of oiol floats on a puddle of water. what is the minimum thickness of the oil needed to completely reflect blue light
Answers
The minimum thickness of the oil needed to completely reflect blue light is approximately 160 nanometers.
It's important to provide a concise answer, so I'll keep my response brief and focused on the essential information.
To find the minimum thickness of the oil needed to completely reflect blue light, we can use the thin-film interference formula:
t = (mλ) / (2n)
where:
- t is the thickness of the oil layer
- m is the order of interference (minimum m = 1 for complete reflection)
- λ is the wavelength of the blue light
- n is the refractive index of the oil
Blue light has a wavelength of approximately 450 nm (nanometers). The refractive index of oil depends on the specific type, but it generally ranges from 1.4 to 1.5.
Using the formula and assuming the minimum order of interference (m = 1) and the lower end of the refractive index range (n = 1.4), we can calculate the minimum thickness of the oil layer:
t = (1 * 450 nm) / (2 * 1.4)
t ≈ 160 nm
Therefore, the minimum thickness of the oil needed to completely reflect blue light is approximately 160 nanometers.
To know more about blue light visit:
https://brainly.com/question/32254285
#SPJ11
2. Choose the atom from each of the following pairs with the greater ionization energy: a. Be and Ba b. Al and Ar c. Ca and Cl
Answers
Considering the definition of ionization energy, the highest ionization energy belongs to the element:
a. Be
b. Ar
c. Cl
Electrons are held in atoms by their attraction to the nucleus, which means that energy is needed to remove an electron from the atom.
You should keep in mind that the electrons of the last layer are always lost, because they are the weakest attracted to the nucleus.
Ionization energy, also called ionization potential, is the necessary energy that must be supplied to a neutral, gaseous, ground-state atom to remove an electron from an atom. When an electron is removed from a neutral atom, a cation with a charge equal to +1 is formed.
In a group, the ionization energy increases upwards because when passing from one element to the bottom, it contains one more layer of electrons. Therefore, the valence layer electrons, being further away from the nucleus, will be less attracted to it and it will cost less energy to pluck them.
In the same period, in general, it increases as you shift to the right. This is because the elements in this way have a tendency to gain electrons and therefore it will cost much more to tear them off than those on the left which, having few electrons in the last layer will cost them much less to lose them.
Considering all the above, from each of the pairs, the highest ionization energy belongs to the element:
a. Be
b. Ar
c. Cl
Learn more:
brainly.com/question/16243729?referrer=searchResults brainly.com/question/11623163?referrer=searchResults brainly.com/question/1602374?referrer=searchResultsWhich of the following is an example of a nonmetal that is solid at room
temperature?
nitrogen
carbon
oxygen
bromine
Answers
Answer:
Carbon
Explanation:
At room temperature20–22 °C (68–72 °F), nitrogen and oxygen are gases, while bromine is a liquid.
BALANCED WORD EQUATION Aluminium carbonate + hydrochloric acid -->
Answers
Answer: 2Al+6HCl→2AlCl3+3H22Al+6HCl → 2AlCl3 + 3H2
Explanation:
A solution at 25°C is 1.0 x 10^-5 M H3O+. What is the concentration of OH- in this solution
Answers
Answer:
1.0 × 10–9 M OH–
Explanation:
pH = -Log[H+]
pOH = -Log[OH-]
But;
pH + pOH = 14
Therefore;
[H+] + [OH-] = 1.0 × 10^-14 M
Therefore;
[OH-] = 1.0 × 10^-14 M - (1.0 × 10^–5 M)
= 1.0 × 10^-9 M OH–
Sodium hydrogen carbonate and citric acid
Word equation
Answers
Answer:
the right answer is:
Explanation:
How to Balance: NaHCO3 + HC2H3O2 = NaC2H3O2 + CO2 + H2O|
Answer:
NaHCO3 +C6H8O7
Explanation:
these are the formulas of sodium hydrogen carbonate and citric acid
Are you brainly, then answer this, I’ll give you brainliest
The most common reaction that alkane under go is?
Select one:
a. Substitution
b. Addition
c. Decomposition
d. Elimination
Explain please
Answers
Answer:
a.substitution
Explanation:
because one of the hydrogen atoms from the methane is replaced with a bromine atom.Alkanes undergo a substitution reaction with halogens in the presence of light. For example, in ultraviolet light , methane reacts with halogen molecules such as chlorine and bromine. This is a substitution reaction because one of the hydrogen atoms from the methane is replaced by a bromine atom.
In the lab, you looked at speed-time graphs to determine the acceleration of the cart for each of the three fan speeds. What was the acceleration of the cart with Low fan speed
Answers
In the lab, you looked at speed-time graphs to determine the acceleration of the cart for each of the three fan speeds. The acceleration of the cart with Low fan speed is 18 cm/s².
The acceleration of the cart with Low fan speed is :
The acceleration is given as :
a = ( Vf - Vi ) / (t f - t i )
by substituting the value in the acceleration formula , we get:
a = 125 - 0 / 7
a = 125 / 7
a = 18 cm/s²
Thus the acceleration of the cart with the low fan speed is 18 cm/s².
To learn more about acceleration here
https://brainly.com/question/17813119
#SPJ4

What is the best description of the constructive interference of light?
A.) The crest of one wave overlaps with the crest of another.
B.) A mechanical wave meets an electromagnetic wave.
C.) The crest and trough of two waves intersect.
D.) A longitudinal wave meets a transverse light wave.
Answers
Answer: The crest and trough of two waves intersect.
Explanation:
The best description of the constructive interference of light is the crest and trough of two waves intersect.
What is Constructive Interference ?When two waves travel in the same direction and are in phase with each other, their amplitude gets added, and the resultant wave is obtained. Here, the waves are said to have undergone constructive interference.
Constructive interference is a type of interference that occurs at any location along the medium where the two interfering waves have a displacement in the same direction
The best description of the constructive interference of light is the crest and trough of two waves intersect.
To know more about Constructive Interference
https://brainly.com/question/16098226
#SPJ2
if equal masses of ch4(g) and o2(g) are placed in a container, exerting a total pressure of 600 torr, what is the partial pressure of ch4(g) ?
Answers
When equal masses of ch4(g) and o2(g) are placed in a container, exerting a total pressure of 600 torrs, then the partial pressure of ch4(g) will be 300torr
Let, x gm o2 and ch4 are present
Given,
Total Pressure = 600torr
Number of moles of o2 = x/16
Number of moles of Ch4 = x/16
According to the Daltons law of partial pressure,
Pa =Xa*Ptotal
Xa is the mole fraction of a
Xch4 =\(\frac{x/16}{x/16 +x/16}\)
=1/2 = 0.5
Pch4 = 0.5×600 = 300torr
Therefore the partial pressure of ch4 will be 300 torr when a total pressure of 600 torr is applied to the mixture.
Learn more about partial pressure:
https://brainly.com/question/28116497
#SPJ4
hydrogen sulfide reacts with oxygen () to produce sulfur dioxide () and water. how many grams of will be produced if 100 g of react with 100 g of ?
Answers
The 187.9 grams of sulfur dioxide will be produced when 100 grams of hydrogen sulfide react with 100 grams of oxygen.
To solve this problem, we need to balance the chemical equation first:
H2S + O2 -> SO2 + H2O
The balanced equation shows that one mole of hydrogen sulfide reacts with one mole of oxygen to produce one mole of sulfur dioxide and one mole of water.
To determine how many grams of sulfur dioxide will be produced, we need to find out which reactant is limiting and which is in excess.
First, we need to calculate the number of moles of each reactant, using their molar masses:
Molar mass of H2S = 34.08 g/mol
Number of moles of H2S = 100 g / 34.08 g/mol = 2.936 mol
Molar mass of O2 = 32.00 g/mol
Number of moles of O2 = 100 g / 32.00 g/mol = 3.125 mol
According to the balanced equation, the reaction requires one mole of O2 for every one mole of H2S. Since we have more moles of O2 than H2S, oxygen is in excess and hydrogen sulfide is limiting.
Therefore, the amount of sulfur dioxide produced will depend on the amount of hydrogen sulfide present. From the balanced equation, we know that 1 mole of H2S produces 1 mole of SO2. So, the number of moles of SO2 produced will be equal to the number of moles of H2S.
Number of moles of SO2 produced = 2.936 mol
Finally, we can calculate the mass of sulfur dioxide produced using its molar mass:
Molar mass of SO2 = 64.06 g/mol
Mass of SO2 produced = 2.936 mol x 64.06 g/mol = 187.9 g
Click the below link, to learn more about Hydrogen sulphide with oxygen:
https://brainly.com/question/31877319
#SPJ11
Sunlight shining through a clear window it’s Jeremy’s face while he does his homework he uses a large wooden wooden block to cover the window what do the wooden block in the clear window demonstrate
Answers
PLS HELP THIS IS SCIENCE !!
IGNORE THE QUESTIONS I ACCIDENTLY PRESSED ON



Answers
Answer:
10.)14
Explanation:
MARK AS BRAINLEST!!
Answer:
where is the answer??
Please do not just take the points without answering the question.
PLS HELP NOW
which of Newton's laws explains the action-reaction forces?
Newton's third law
Newton's second law
Newton's first law
Answers
Answer:
Newtons third law
Explanation:
Hope this as helpful
2. Explain Phenomena Describe what happens to particle motion and temperature as the metals cool. How does particle motion relate to the thermal energy of the metals?
Answers
Answer:
As the metals cool, the particles slow down, and the temperature decreases. Thermal energy is related to the average kinetic energy of the particles.
Explanation:
Help please :<
I just need the answer for this as quickly as possible.
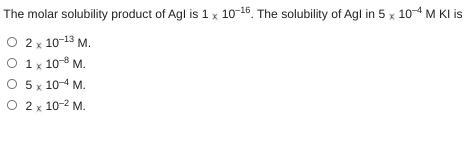
Answers
Answer:
I think its the 3th one
Explanation:
answer the question briefly: How can atom collapse and why are atoms stable?
Answers
The nuclei of atoms become unstable when the repelling forces of the protons cannot be balanced by the number of neutrons in the nucleus. It then re-arranges itself randomly to a more stable configuration by emitting any of a series of particles. During radioactive decay, an atom does not collapse.
Since an atom is mostly empty space - that is it’s nucleus is relatively distant from the electron shells so, in the presence of extreme forces such as gravity inthe collapse of a large star, the inward pressures on the atom overcome the natural balance of the atomic structure and the ‘empty space’ disappears as nuclei are mashed together by the intense pressures and a neutron star is formed. Under even more external pressure, even the neutron star can collapse to form a black hole.
A loan is being repaid by 2n level payments, starting one year after the loan. Just after the nth payment the borrower finds that she still owe (3/4) of the original amount. What proportion of the next payment is interest?
Answers
A loan is being repaid by \(2n\) level payments, starting one year after the loan. Just after the nth payment the borrower finds that she still owe \((3/4)\)of the original amount. The proportion of the next payment that is interest is \(1 - 2 * (1 - (1 + r)^{(-n)}) / (3 * (1 - (1 + r)^{(-2n)})).\)
Let \(P\) be the original amount of the loan, and let \(x\) be the level payment made at each of the \(2n\) payments. Then the total amount repaid will be \(2nx\). We know that after \(n\) payments, the borrower still owes \((3/4)P\).
Therefore, the amount repaid after \(n\) payments is \((P - (3/4)P) = (1/4)P\). This means that the total amount repaid after the remaining \(n\) payments is \((3/4)P\).
We can set up an equation using the formula for the present value of an annuity:
\(P = x * (1 - (1 + r)^{(-2n)}) / r\)
where \(r\) is the interest rate per payment period (which we will assume is constant), and the first payment is due one year after the loan.
After \(n\) payments, the outstanding balance is \((3/4)P\). We can use the same formula to find the present value of the remaining \(n\) payments, but with \(P\) replaced by \((3/4)P\):
\((3/4)P = x * (1 - (1 + r)^{(-n)}) / r\)
We can rearrange this equation to solve for \(x\):
\(x = (3/4)P * r / (1 - (1 + r)^{(-n)})\)
Now we need to find the proportion of the next payment that is interest. The interest component of each payment is the difference between the total payment and the amount of principal being repaid. The total payment is \(x\), and the amount of principal being repaid is:
\((3/4)P * r / (1 - (1 + r)^{(-n)})\).
So the proportion of the next payment that is interest is:
Interest component / Total payment
\(= (x - (3/4)P * r / (1 - (1 + r)^{(-n)})) / x\\= 1 - (3/4)P * r / (x * (1 - (1 + r)^{(-n)}))\\= 1 - (3/4)P / (2nx * (1 - (1 + r)^{(-n)}))\\\)
We can simplify this expression by using the equation we derived earlier for \(x\):
\(1 - (3/4)P / (2nx * (1 - (1 + r)^{(-n)}))\\= 1 - (3/4)P / ((3/2)P * r / (1 - (1 + r)^{(-n)}) * (1 - (1 + r)^{(-2n)}))\\= 1 - 2 * (1 - (1 + r)^{(-n)}) / (3 * (1 - (1 + r)^{(-2n)}))\)
So the proportion of the next payment that is interest is:
\(1 - 2 * (1 - (1 + r)^{(-n)}) / (3 * (1 - (1 + r)^{(-2n)})).\)
Learn more about Interest at
brainly.com/question/30393144
#SPJ4
Can someone please help me with this!!!
( I chose to stop using water bottles )

Answers
Answer:
Flushing goldfish down the toliet
Explanation:
This would help the planet because goldfish are a very evasive species that has been harming our natural enviroments lakes and rivers. Ever since people have been flushing them down the toliet including me since I've had over six they have been finding their ways into our ecosytem and damaging nature. You may think that goldfish are a cute and small pet species to keep in a fish tank but the fish can grow in the right conditons up to 16.1 inches long and can top over five pounds in the wild. Unfortunately most goldfish never thrive like this in fish tanks due to nasty inadequate housing and feeding conditions. And most do not live up to their expected life span in the wild of 15 to even thirty years due to their living conditons. Did you know, goldfish will actually jump out of their tank in a final desperate search for better living conditions and enviroment to live in to meet their physiological needs. But lets get back to the problem so don't be to quick to feel sorry for them, when people flush them down the toliet they repopulate somehow in our lakes and rivers. Even through, it is not clear how they got there, which the suspected culprit is the sewer system. They threaten the natural enviroment tho because they are obnivores and they eat a mixtures of plants and animal specimen. You are probrably thinking why is this a problem, well its because goldfish are particullary greedy eaters and will eat, eat, and feast on the natural envrioment they infestated. And the waste they leave on the enviroment will promote the growth of algae which could cause an extent of enviromental damage. Of course goldfish are not impacted by this algae growth for adaptation reasons which shield them. This is not the main problem tho the main problem of this is that female goldfish can produce up to 1000 eggs at a time. And to make things worse unlike other fish species where the parents of the eggs leave the eggs for predaetors and well the elements of nature the goldfish instead guard their nest foriciously from predaetors that eat fish eggs in which effectively shields the populations young from danger. And to just add to the chaos the goldfish sadly have no real natural predaetor in the unitedstates which just causes more problems since they have no one killing them. And did I mention that they could live up to a real possibility of thirty years. And that they are forocious eaters that could eat a variety of types of foods. All of these facts in the end lead to two things first stop flushing your pet goldfish down the toliet and secondly now you know how golfish impact our planet.
Give brainliest please it took me over thirty minutes of thinking!
Using the periodic table determine the number of neutrons in 16O
Answers
We often denote oxygen as 16O8 where 16 is the mass number and 8 is the atomic number.
number of neutrons=16-8=8
A weather balloon calibrated at 0.00 °C to have a volume of 20.0 L has
what volume at -40.0 °C assuming pressure is held constant?
Answers
Answer:
17.1 Liters
Explanation:
It's a gas law question (more specifically a Charles's Law question). Formula is V1/T1 = V2/T2. You're given V1 and T1 and T2. However, in order to use the equation, temperature needs to be in Kelvins (by subtracting the degrees C from 273) for the numbers to work (among other reasons, the 0 degrees C will always give you an answer of zero or undefined). Placing all temps in kelvins makes the answers come out right. So 20L/273K = xL/233K gives you the answer when you cross-multiply.
Considering the Charles's law, a weather balloon calibrated at 0.00 °C to have a volume of 20.0 L has 17.07 L at -40.0 °C, assuming pressure is held constant.
The gas laws are a set of chemical and physical laws that allow determining the behavior of gases in a closed system. The parameters evaluated in these laws are pressure, volume, temperature and moles.
Charles's law is one of the gas laws. It relates the volume and the temperature of a certain quantity of ideal gas, kept at a constant pressure.
This law states that, at constant pressure, the volume of a gas is directly proportional to its temperature. In other words, for a given sum of gas at constant pressure, as the temperature increases, the volume of the gas increases, and as the temperature decreases, the volume of the gas decreases.
Mathematically, Charles's law says that the quotient that exists between the volume and the temperature will always have the same value:
\(\frac{V}{T}=k\)
Being an initial state 1 and a final state 2, it is true:
\(\frac{V1}{T1}=\frac{V2}{T2}\)
In this case, you know:
V1= 20 LT1= 0 C=273 KV2= ?T2= -40 C= 233 KReplacing:
\(\frac{20 L}{273 K}=\frac{V2}{233 K}\)
Solving:
\(V2=233 K x\frac{20 L}{273 K}\)
V2=17.07 L
Finally, a weather balloon calibrated at 0.00 °C to have a volume of 20.0 L has 17.07 L at -40.0 °C, assuming pressure is held constant.
Learn more:
https://brainly.com/question/4147359?referrer=searchResultsDoes changing the number of protons change the identity of the element you have built ?
Answers
Answer: Yes.
Explanation:
the number of protons in the nucleus determines an element's identity. Chemical changes do not affect the nucleus, so chemical changes cannot change one type of atom into another. The number of protons in a nucleus does change sometimes, however. The identity of the atom, therefore, changes.
cisplatin, pt(nh3)2cl2, an anticancer agent used for the treatment of solid tumor, is prepared by the reaction of ammonia, nh3, with potassium tetrachloroplatinate, k2ptcl4: k2ptcl4 nh3 pt(nh3)2cl2 kcl potassium cisplatin tetrachloroplatinate a) balance the above chemical equation. b) in an experiment 5.00 g of potassium tetrachloroplatinate, k2ptcl4, reacted with 5.00 g of ammonia, nh3: i) which reactant is limiting? ii) calculate the mass of cisplatin produced, (theoretical yield). iii) if a student obtained 1.60 g of cisplatin, calculate the percent yield.
Answers
(a)K₂PtCl₄ + 2NH₃ → Pt(NH₃)₂Cl₂ + 2KCl is balanced chemical equation.
(b) The limiting reactant is K₂PtCl₄ because it has fewer moles. Mass of Pt(NH₃)₂Cl₂ (theoretical yield) = 3.60 g. The percent yield of cisplatin is 44.4%.
a) Balanced chemical equation:
K₂PtCl₄ + 2NH₃ → Pt(NH₃)₂Cl₂ + 2KCl
b) Given:
Mass of K₂PtCl₄= 5.00 g
Mass of NH₃= 5.00 g
i) To determine the limiting reactant, we need to compare the moles of each reactant. First, we calculate the moles of K₂PtCl₄ and NH₃:
Molar mass of K₂PtCl₄ = 2 × (39.10 g/mol of K) + 195.08 g/mol of Pt + 4 × (35.45 g/mol of Cl) = 415.27 g/mol
Molar mass of NH₃ = 14.01 g/mol of N + 3 × (1.01 g/mol of H) = 17.03 g/mol
Moles of K₂PtCl₄ = Mass / Molar mass = 5.00 g / 415.27 g/mol = 0.0120 mol
Moles of NH₃ = Mass / Molar mass = 5.00 g / 17.03 g/mol = 0.293 mol
The ratio of K₂PtCl₄ to NH₃ in the balanced equation is 1 ratio 2. Therefore, the limiting reactant is K₂PtCl₄ because it has fewer moles.
ii) The molar ratio between K₂PtCl₄ and Pt(NH₃)₂Cl₂ is 1 ratio 1, which means that for every mole of K₂PtCl₄, one mole of Pt(NH₃)₂Cl₂ is produced. Therefore, the mass of Pt(NH₃)₂Cl₂ produced is equal to the molar mass of Pt(NH₃)₂Cl₂.
Molar mass of Pt(NH₃)₂Cl₂ = 195.08 g/mol of Pt + 2 ×(17.03 g/mol of N + 3 ×1.01 g/mol of H) + 35.45 g/mol of Cl = 300.05 g/mol
Mass of Pt(NH₃)₂Cl₂ (theoretical yield) = Moles of limiting reactant (K₂PtCl₄) × Molar mass of Pt(NH₃)₂Cl₂
= 0.0120 mol × 300.05 g/mol = 3.60 g
iii) Percent yield is calculated using the formula: Percent Yield = (Actual Yield / Theoretical Yield) × 100%
Given:
Actual Yield = 1.60 g
Theoretical Yield = 3.60 g
Percent Yield = (1.60 g / 3.60 g) × 100% = 44.4%
Therefore, the percent yield of cisplatin is 44.4%.
To know more about cisplatin:
https://brainly.com/question/30439227
#SPJ4
the equivalence points of the titration curves were not in the same ph range. explain
Answers
The equivalence points of the titration curves were not in the same pH range due to differences in the acid and base strengths of the titrants used in the titration reactions.
In a titration, the equivalence point is reached when the stoichiometrically equivalent amounts of acid and base have reacted. At this point, the solution is neutral, and the pH is determined by the nature of the salt formed.
The pH range at the equivalence point depends on the acid and base used in the titration. Strong acids and strong bases have a high degree of dissociation in water, resulting in a large concentration of ions and a pH close to 7 at the equivalence point.
On the other hand, weak acids and weak bases have a lower degree of dissociation, leading to lower concentrations of ions and a pH that deviates from neutrality at the equivalence point. The pH range at the equivalence point for weak acids and weak bases can vary depending on their dissociation constants and concentrations.
Therefore, if different titrants with different acid and base strengths are used in titration reactions, the equivalence points may not occur in the same pH range. The acid-base properties of the titrants influence the pH at the equivalence point, resulting in variations in the pH range observed in the titration curves.
To know more about titration refer here:
https://brainly.com/question/31483031#
#SPJ11
What is the mass of 2. 23x1023 atoms of sulphur
Answers
Mass of 2.23x10²³ atoms of sulphur with molar mass of 32.07 grams per mole is equals to the 2.65 g per mole.
Avogadro's number, is a constant number of units in one mole of any substance (may be defined as its molecular weight in grams), equal to 6.02214076 × 10²³. The units represents electrons, atoms, ions, or molecules, depending on the nature of the substance and the character of the reaction. We have 2.23× 10²³ atoms of sulpher. We have to determine the mass of these atoms. Now, one mole of sulphur is 6.02214076 × 10²³ atoms or molecules.
Molar mass of sulphur = 32.07 grams/mol
6.02214076 × 10²³ atoms or molecules = 1 mole
1 atom =\( \frac{ 1}{6.02214076 × 10²³}\)
So, 2.23× 10²³ atoms of sulphur = \(2.23× 10²³ × \frac{ 1}{6.02214076 × 10²³}\) moles. Using molar mass formula, Molar mass = mass of substance divided by number of moles of substance.
=> Mass of sulphur = \(32.07 ×2.23× 10²³ × \frac{ 1}{6.02214076 × 10²³} g\\ \)
= 2.65 g
Hence, required value is 2.65 g per mole.
For more information about molar mass , visit :
https://brainly.com/question/30459977
#SPJ4
What is the limiting reagent in this experiment, sodium bromide or 1-butanol?
Answers
the balanced equation of the reaction :NaBr + C4H9OH → C4H9Br + Na OH Sodium Bromide (NaBr) is the limiting reagent in the experiment, not 1-butanol.
The limiting reagent in the experiment between sodium bromide and 1-butanol is sodium bromide.What is a limiting reagent ?A limiting reagent is a reactant in a chemical reaction that restricts the yield of the product. It means the reaction can't go on forever because the reagents are consumed up. In general, the limiting reagent determines the amount of products that can be produced during a reaction .In the given chemical reaction between sodium bromide and 1-butanol, it is essential to know which reactant is the limiting reagent. the balanced equation of the reaction :NaBr + C4H9OH → C4H9Br + Na OH Sodium Bromide (NaBr) is the limiting reagent in the experiment, not 1-butanol.To identify the limiting reagent, you need to know the balanced chemical equation, the amounts or concentrations of the reactants, and their stoichiometric ratios. With that information, we can compare the actual amounts of each reactant to their stoichiometric ratios to determine which one will be completely consumed and thereby limit the reaction.
to know more about stoichiometric, visit
https://brainly.com/question/14935523
#SPJ11
Which choice is not true of a liquid in a glass capillary with a convex meniscus?a. The liquid has strong cohesive forces.b. The liquid level will be lower inside the capillary when a capillary is inserted into a bowl of the liquid.c. The liquid will have a convex meniscus as it moves in the capillary.d. The behavior of the liquid is driven by strong interactions with the capillary glass.
Answers
The correct answer is the liquid level will be lower inside the capillary when a capillary is inserted into a bowl of the liquid.
When a liquid is placed in a glass capillary with a convex meniscus, the behavior of the liquid is driven by strong cohesive forces between the liquid molecules and strong interactions with the capillary glass. As a result, the liquid will have a convex meniscus, meaning that the surface of the liquid will be curved outward. This is due to the forces acting on the liquid in the capillary. However, when a capillary is inserted into a bowl of the liquid, the liquid level inside the capillary will be higher than the liquid level outside the capillary. This is because the cohesive forces between the liquid molecules are stronger inside the capillary, where the liquid is in contact with the walls of the capillary, than they are outside the capillary. As a result, the liquid will be pulled up the walls of the capillary, causing the liquid level inside the capillary to be higher than the liquid level outside the capillary. This is opposite to what is stated in choice b, which is why it is the correct answer.
to know more about meniscus-
https://brainly.com/question/28013459
#SPJ4