What happens to the length of the day during the solstices.
Answers
The length of the day varies during the solstices.
The solstices happen twice every year, once in June and again in December. The Northern Hemisphere experiences the longest day of the year and the shortest night during the summer solstice in June. On the other hand, the Southern Hemisphere experiences its shortest day and longest night during the June solstice, which is its winter solstice.
During the December solstice, the opposite occurs, with the Northern Hemisphere experiencing the shortest day and longest night of the year, while the Southern Hemisphere has its longest day and shortest night. At the equator, the length of the day and night is almost the same throughout the year. The length of the day varies as you travel farther north or south from the equator, owing to the Earth's tilted axis.
The length of the day varies during the solstices, with the Northern Hemisphere experiencing the longest day and shortest night during the summer solstice, while the Southern Hemisphere experiences its shortest day and longest night during the June solstice.
To know more about solstices, visit:
https://brainly.com/question/29085019
#SPJ11
Related Questions
what volume is occupied by 20.3 gg of argon gas at a pressure of 1.27 atmatm and a temperature of 429 kk ?
Answers
At 1.27 atm and 429 K, the volume occupied by 20.3 g of argon gas is roughly equal to 10.9 L.
We must apply the ideal gas law, which connects a gas's pressure, volume, mass (measured in moles), and temperature, to this issue. The molar mass of argon can be used to convert its mass to moles, and the ideal gas law can be used to solve for volume. Converting units, such as pressure in pascals, volume in cubic metres, and temperature in kelvins, is necessary for the computation to guarantee that they are in the proper SI units. After determining the volume, we can convert the result to litres to make understanding easier. The end result is roughly 10.9 L.
Learn more about volume here:
https://brainly.com/question/13338592
#SPJ4
what mass (in grams) of NH4Cl is needed to prepare 350 mL of a 0.25 M ammonium chloride solution
Answers
Answer:
4.70 grams of NH4Cl is needed to prepare 350 mL of a 0.25 M ammonium chloride solution.
We need approximately 4.68 grams of NH4Cl to prepare a 0.25 M ammonium chloride solution with a volume of 350 mL.
To determine the mass of NH4Cl needed to prepare the solution, we us use the formula:
m=M x V x MM ... (i)
where,
m= mass in grams
M=molarity of solution
MM= molar mass of compound
V= volume in litres
The number of moles of NH4Cl needed can be calculated using:
Moles = Molarity x Volume ...(ii)
Moles = 0.25 mol/L x 0.350 L
Moles = 0.0875 mol
Hence we can replace M x V with number of moles in equation i.
The molar mass of NH4Cl is :
Molar mass of NH4Cl = (1 x 14.01 g/mol) + (4 x 1.01 g/mol) + (1 x 35.45 g/mol)
Molar mass of NH4Cl = 53.49 g/mol
We have all the variables
Putting them in equation i.
Hence,
Mass (g) = Moles x Molar mass
Mass (g) = 0.0875 mol x 53.49 g/mol
Mass (g) = 4.68 g
Therefore, you would need approximately 4.68 grams of NH4Cl to prepare a 0.25 M ammonium chloride solution with a volume of 350 mL.
To learn more about Stoichiometry,
https://brainly.com/question/16060223
Where on the periodic table are you most likely to find elements that do not react with other elements A) group 1 B) group 2 C) group 17 D) group 18
Answers
Answer:
d) group 18
Explanation:
Group 18 on the periodic table consists of noble gasses, which do not chemically interact with other elements.
Directions: Answer the following questions in your own words using complete sentences. Do not copy and paste from the lesson or the internet.
1. How are the forest biomes classified?
2. Describe each of the forest biomes.
3. Name some environmental concerns about the forest biomes?
4. What is one main contribution forests make to the environment?
5. Conduct research into one forest biome and identify a particular forest. Identify any environmental issues connected to your forest biome. Are there ways to solve some of those environmental concerns?
Answers
The supporting initiatives that focus on reforestation and restoration can help restore damaged areas and promote the resilience of the forest ecosystem.
Forest biomes are classified based on their geographical location, climate, and dominant plant species. The main classifications of forest biomes include tropical rainforests, temperate forests, and boreal forests.
Tropical rainforests are found in regions near the equator and are characterized by high temperatures, abundant rainfall, and a dense canopy of tall trees. They are known for their incredible biodiversity and complex ecosystems.
Temperate forests are found in regions with moderate climates and distinct seasons. They have a mix of deciduous and evergreen trees, and their foliage changes color in autumn. These forests support a wide range of plant and animal species.
Boreal forests, also known as taiga, are found in subarctic regions and are characterized by long, cold winters and short summers. They consist mainly of coniferous trees like spruce, pine, and fir. Boreal forests are essential for regulating global climate and support unique wildlife adapted to harsh conditions.
Some environmental concerns about forest biomes include deforestation, habitat loss, biodiversity loss, climate change, and illegal logging. Deforestation, primarily driven by human activities such as agriculture, logging, and infrastructure development, leads to the destruction of forests and the loss of wildlife habitats. This loss of biodiversity can have far-reaching ecological consequences.
Climate change also poses a threat to forest biomes, as it can alter precipitation patterns, increase the frequency of wildfires, and disrupt ecosystems. Illegal logging exacerbates these issues by contributing to deforestation and forest degradation.
One main contribution forests make to the environment is their role in carbon sequestration. Forests absorb carbon dioxide from the atmosphere through photosynthesis and store it in their biomass and soil. This helps mitigate climate change by reducing the concentration of greenhouse gases in the atmosphere.
The Amazon Rainforest, located in South America, is a prime example of a forest biome. It is the largest tropical rainforest in the world and plays a crucial role in global climate regulation and biodiversity conservation. However, the Amazon Rainforest faces significant environmental issues, including deforestation, illegal logging, and land conversion for agriculture.
Deforestation in the Amazon is primarily driven by the expansion of agricultural activities, such as cattle ranching and soybean production. This results in habitat loss for countless plant and animal species, including endangered ones. The clearing of land also releases large amounts of stored carbon into the atmosphere, contributing to climate change.
Solving the environmental concerns in the Amazon Rainforest requires a multi-faceted approach. It involves implementing stricter regulations and enforcement against illegal logging and land encroachment, promoting sustainable agricultural practices, supporting local communities, and increasing international efforts to protect and conserve this vital ecosystem.
For more such questions on ecosystem
https://brainly.com/question/26046675
#SPJ11
giving brainly if correct!!! answer as many as possible

Answers
Molarity shows the quantitative relationship between - number of moles of solute and mass of solvent - number of moles of solute and liters of solution - number of moles of solute and the number of moles of solvent - number of moles of solute and sum of moles of solvent and solute Incorrect
Answers
Molarity shows the quantitative relationship between b. the number of moles of solute and liters of solution.
Molarity, also known as molar concentration, is a unit of concentration that refers to the number of moles of a solute that is present in one liter of a solution. It's a measure of the amount of solute in a solution that is proportional to the volume of the solution. The molarity formula is given by;Molarity (M) = number of moles of solute ÷ volume of solution in litersMolarity is expressed in units of moles per liter (mol/L) or millimoles per liter (mmol/L).
Therefore, the molarity of a solution is proportional to the number of moles of solute that is present in a given volume of solution. It is used in chemistry to calculate the amount of solute needed to prepare a given volume of solution or to determine the concentration of a solution.
Learn more about solution at:
https://brainly.com/question/30198131
#SPJ11
what is the ionic charge lf this atom
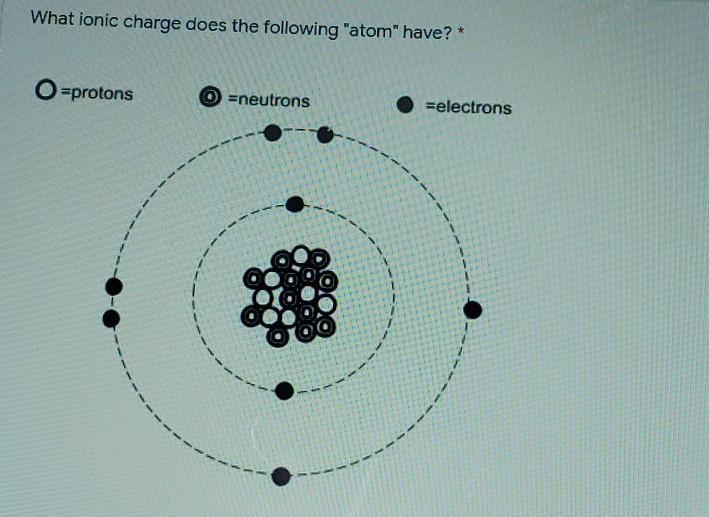
Answers
Answer:
An ionic charge of 2-
Explanation:
The number of electrons is 8 and the number of protons is 2, subtract the two and the difference is the ionic charge. Hope this helps! :)
Answer:
This answer would be 2
Explanation:
The way that I have always done it is you put protons in tally marks (without crossing) over the top of the neutrons and mark them out one by one and whichever one that you have leftover that is your charge.
Hope this helps!
Which of these is an example of mutualism
Answers
Answer:
B.
Explanation:
Mutualism is the idea that both organisms benefit from an interaction. In example B, the honeybee benefits because they receive nectar, while the flower benefits in being pollinated.
You set up a radio transmitter that operates by moving electric charges through a wire in a north-south direction. If you turn the transmitter so that the charges move in a east-west direction, what property of the radio waves will change
Answers
When you turn the transmitter so that charges move in a east-west direction, the polarization of the resulting electromagnetic waves will change.
What is polarization?Polarization is the orientation of the electric field vector of an electromagnetic wave. In electromagnetic wave generated by north-south current in a wire, electric field vector will be oriented in an east-west direction. If the current in the wire is moving in east-west direction, the electric field vector of resulting electromagnetic wave will be oriented in a north-south direction.
So, if you change the direction of electric charges in the wire, polarization of the resulting electromagnetic waves will change.
To know more about polarization, refer
https://brainly.com/question/3092611
#SPJ1
Why does water roll off of dry ice?
Answers
Calculate the absolute error and the percent error for the following data sets.
Make sure to show your work and include the correct units with your final answer. You final answer should show correct sig figs as well.
A) A student measured the boiling point of water to be 209°F. The accepted value is 212°F.
B) A bowling ball weighs 8 lbs. You decide to test your bathroom scale and you measure that ball weighing 7.35 lbs.
Answers
(A) A student measured the boiling point of water to be 209°F . the accepted value is 212°F. the absolute error is 3°F and the percent error is 1.41 5
(B) a bowling ball weighs 8 lbs. you decide to test your bathroom scale and you measure that ball weighing 7.35 lbs . the absolute error is 0.65lbs and the percent error is 8.12 %
(A) The actual value = 212°F and The observed value = 209°F
The absolute error = | actual value - observed value |
= | 212 °F - 209 °F |
= 3 °F
percent error = ( | actual value - observed value | /actual value ) × 100 %
= ( 3 / 212 ) × 100 %
= 1.41 %
(B) The actual value = 8 lbs
The observed value = 7.35 lbs
The absolute error = | actual value - observed value |
= | 7.35 - 8 |
= 0.65 lbs
percent error = ( | actual value - observed value | /actual value ) × 100 %
= ( 0.65 / 8 ) × 100%
= 8.12 %
Thus, (A) A student measured the boiling point of water to be 209°F . the accepted value is 212°F. the absolute error is 3°F and the percent error is 1.41 %
(B) a bowling ball weighs 8 lbs. you decide to test your bathroom scale and you measure that ball weighing 7.35 lbs . the absolute error is 0.65 lbs and the percent error is 8.12 %
To learn more about absolute error and percent error here
https://brainly.com/question/20428218
#SPJ1
Why should we not were
synthetic clothes while working
in kitchen ?
Answers
Answer:
Synthetic clothes can easily build up static electricity. It also easily can catch up on fire and when it does, it melts and can fuse to your skin.
Explanation:
Answer:
Explanation:
Synthetic fibres like Polyester catches fire very easily and melts. After melting It sticks to the body of the person wearing it causing severe burns. Hence it is advised not wear synthetic clothes while working in the Kitchen. Its advised to wear Apron or cotton clothes while cooking in Kitchen.
how many moles of water vapor are in 12510 l of gas over water at 27°c? (in a hotel pool room, for example.)
Answers
There are 18.3 moles of water vapor in 12510 L of gas over water at 27°C.
To calculate the number of moles of water vapor in 12510 L of gas over water at 27°C, we need to use the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
In this case, we know the volume (V) of the gas, but we need to determine the pressure (P) of the water vapor in the gas. We can use the fact that the gas is over water to find the partial pressure of water vapor using the vapor pressure of water at 27°C, which is 3.67 kPa.
therefore, the partial pressure of water vapor in the gas is 3.67 kPa.
Now, we can rearrange the ideal gas law to solve for n:
n = PV/RT
where P is the partial pressure of water vapor, V is the volume of the gas, R is the gas constant (8.31 J/mol·K), and T is the temperature in Kelvin (300 K).
Substituting the values, we get:
n = (3.67 kPa) x (12510 L) / (8.31 J/mol·K x 300 K)
n = 18.3 moles of water vapor
For more question on moles click on
https://brainly.com/question/29367909
#SPJ11
"The Periodic Table Turns 150: Is the Best Yet to Come?" ChemMatters, February/March 2019
Student Reading oilesu
Comprehension Questions
Name
Directions: Use the article to answer the questions below.
1. What was Dmitri Mendeleev's dream that reportedly was the start of his periodic table?
2. What is periodicity?
3. How did (a) Antoine Lavoisier, (b) Johann Döbereiner, and (c) John Newlands attempt to
organize the elements?
Answers
1) The dream of Mendeleev concerned the arrangement of the elements by mass
2) Periodicity is the changing properties of the elements
3)
Antoine Lavoisier - Arranged the elements by their properties
Johann Döbereiner - Arranged the elements into triads
John Newlands - Arranges the elements according to the order of increasing atomic mass
What is the periodic table?We define the periodic table as the arrangement of the elements in order of increasing atomic numbers. We know can see that several attempts have been made at the systematization of the elements and the approaches to the problem have taken different dimensions. In a dream, Mendeleev saw the elements line up in order of atomic masses and there was a visible pattern.
The term periodicity has to do with the regular repeating property of the elements and this can be seen when we follow the properties of the elements as they change down the group and across the period.
Learn more about periodic table:https://brainly.com/question/11155928
#SPJ1
What is the volume, in liters, of 1.4 moles of CO2 gas at STP?
Answers
Answer:
22.4 L.
Explanation:
c-14 (radiocarbon) is radioactive and decays into n-14 with a half-life of 5730 years. assume a rock starts with 1000 atoms of c-14. if the rock is 5730 years old, how many c-14 atoms should be left?
Answers
250 , C-14 atoms should be left, if the rock is 5730 years old. C-14 (radiocarbon) is radioactive and decays into n-14 with a half-life of 5730 years. assume a rock starts with 1000 atoms of C-14.
Radiation-emitting radiocarbon substances are referred to as radioactive . A radionuclide decays into a different atom known as a decay product. Until the atoms achieve a stable state and stop being radioactive, the radiocarbon continue to change into new decay products. C degrades through a process known as beta decay. One of the neutrons in the radiocarbon atom turns into a proton during this process, which results in the decay of a 14C atom into a 14N atom. By adding one more proton to the atom, this results in the formation of a nitrogen atom rather than a radiocarbon atom.
learn more about decay here:
https://brainly.com/question/9615302
#SPJ4
Which equation is balanced?
CH4 + O2 ⟶ CO2 + H2O
Mg + 2HCl ⟶ MgCl2 + H2
Mg + P4 ⟶ Mg3P2
Answers
Answer:
A I think
Explanation:
im not sure so do with that what you will
a chemical company makes a silver by reacting silver nitrate would see the company needs to make 800 g of pure silver for a client they have 300 g of zinc and 600 g of silver nitrate will they be able to make enough silver to fill the order
Answers
Answer
Explanation
Given that:
The mass of pure silver needed = 800 g
Mass of zinc = 300 g
Mass of silver nitrate = 600 g
What to find:
Will the mass of zinc and silver nitrate be able to make 800 g of pure silver.
Step-by-step solution:
Step 1: Write the balanced equation for the reaction.
Zn + 2AgNO₃ → 2Ag + Zn(NO₃)₂
Step 2: Determine the moles of the reactants.
Using the mole formula, the moles of the reactants will be:
\(\begin{gathered} Moles\text{ }of\text{ }Zn=\frac{Mass}{Molar\text{ }mass}=\frac{300g}{65.38g\text{/}mol}=4.5886\text{ }mol \\ \\ Moles\text{ }of\text{ }AgNO_3=\frac{600g}{169.87g\text{/}mol}=3.5321\text{ }mol \end{gathered}\)Step 3: Determine the moles of pure silver produced.
Using the mole ratio of Zn to AgNO₃ in the equation and the moles in step 2, we
Low-beam headlights must show objects _______ feet ahead.
Answers
Answer:
150 feet
Explanation:
Balance Equation:__H2O + __ F2 > __HF + __O2
Answers
Explanation:
We have to balance the following equation:
__ H₂O + __ F₂ -------> __ HF + __ O₂
First we have to determine the number of atoms of each element that we have on both sides of the equation.
__ H₂O + __ F₂ -------> __ HF + __ O₂
O: 1 O: 2
H: 2 H: 1
F: 2 F: 1
We have 2 atoms of O on the right side and 1 atom of O on the left side. To balance the O atoms we can change the coefficient for H₂O and write a 2 in front of it.
2 H₂O + __ F₂ -------> __ HF + __ O₂
O: 2 O: 2
H: 4 H: 1
F: 2 F: 1
Then we have 4 atoms of H on the left and 1 atom of H on the right side of the equation. We can change the coefficient for HF to balance the H atoms.
2 H₂O + __ F₂ -------> 4 HF + __ O₂
O: 2 O: 2
H: 4 H: 4
F: 2 F: 4
And finally we have 2 atoms of F on the left and 4 atoms of F on the right. We can change the coefficient for F₂ and write a 2 there.
2 H₂O + 2 F₂ -------> 4 HF + __ O₂
O: 2 O: 2
H: 4 H: 4
F: 4 F: 4
The equation is balanced.
Answer: 2 H₂O + 2 F₂ -------> 4 HF + O₂
Help what is the answer?

Answers
The specific heat of mercury calculated from her data is 0.13 J/g°C.
Specific heat is a measure of how much energy is required to heat a substance. This is the amount of energy (in joules) required to heat 1 gram of a substance by 1°C. Different substances have different specific heats.
To answer this question, use the heat slaughter formula:
Q =mCΔT
In the question:
Heat energy: Q = 30.1 J
Mass of mercury: m = 12.5 g
T1 = 21.2
T2 = 39.6°C
Temperature:
ΔT = T2 –T1
∆T = 39.6 – 21.2
∆T = 18.4°C
The specific heat of mercury is?
Q =mCΔT
C = Q/mΔT
C = 30.1/12.5 x 18.4
C =30.1/230
C = 0.13 J/g°C
So, the specific heat of mercury is 0.13 J/g°C.
Question:
In the laboratory a student finds that it takes 30.1 Joules to increase the temperature of 12.5 grams of liquid mercury from 21.2 to 39.6 degrees Celsius.
The specific heat of mercury calculated from her data is_____J/g°C.
Learn more about the specific heat at https://brainly.com/question/29792498
#SPJ1
Can someone help me with this chem question?

Answers
Answer:
Malleable
Explanation:
C - Good thermal insulator
Explanation:
Metals are ductile, malleable, and are good electrical conductors. They are also good conductors of heat, but that doesn’t mean they make good thermal insulators.
what chemicals help reduce a change in ph when acids are added to a solution?
Answers
Chemicals that help reduce a change in pH when acids are added to a solution are called buffers. Buffers are substances that are capable of resisting changes in pH by accepting or donating protons (H+ ions) in order to maintain a relatively stable pH.
Buffers typically consist of a weak acid and its conjugate base (or a weak base and its conjugate acid). When an acid is added to a buffered solution, the weak base component of the buffer can accept the additional H+ ions, minimizing the increase in pH. Conversely, when a base is added, the weak acid component of the buffer can donate H+ ions, minimizing the decrease in pH. Common examples of buffer systems include acetic acid/acetate buffer (found in vinegar), phosphate buffer (used in biological systems), and bicarbonate buffer (important in maintaining blood pH).In summary, buffers help reduce the change in pH when acids are added to a solution by accepting or donating protons to maintain the pH within a relatively narrow range.
To know more about protons, click here https://brainly.com/question/30895149
#SPJ11
For the hydrogen atom the electron is separated from the nucleus by the distance r≈5.28× 10
−11
m, use ∣q∣=1.6×10
−19
C for both the electron and nucleus, and respective masses of m
e
=9.1×10
−31
kg and m
n
≈m
p
=1.67×10
−27
kg. Recall that the respective electric and gravitational force is given by,
F
e
=
4πϵ
0
1
r
2
q
e
q
n
, and
F
g
=G
r
2
m
e
m
n
,
with G=6.67×10
−11
m
3
⋅kg
−1
⋅s
−
2, the universal gravitational constant. Determine the ra
Answers
Given data,
∣q∣=1.6×10−19 Cm
e=9.1×10−31 kgm
n≈m
p=1.67×10−27
kgr≈5.28×10−11 m
Formula:
F e = 4πϵ 0 1 r2q e q n
F g = G r 2m e m n
Given that,
∣q∣=1.6×10−19 C,
r≈5.28×10−11 m,
m e=9.1×10−31 kg
m n≈m p=1.67×10−27 kg.
F e = 4πϵ 0 1 r2q e q
n = 9 × 10 9 N.
m2C−2 × 1 × (1.6 × 10−19 C)2 / (5.28 × 10−11 m)2
= 8.2 × 10−8 N
Similarly,
F g = G r 2m e m
n = 6.67 × 10−11 Nm2kg−2 × (1.67 × 10−27 kg) × (9.1 × 10−31 kg) / (5.28 × 10−11 m)2
= 8.2 × 10−8 N.
The radius of the hydrogen atom is the radius at which the attractive electric force between the proton and the electron equals the gravitational force, or,
FE = FG8.2 × 10−8 N = 8.2 × 10−8 N 5.28 × 10−11 m
= (4πε₀) (1.6 × 10−19 C)2 / r2 G (1.67 × 10−27 kg) (9.1 × 10−31 kg)
Simplifying,
r3 = (4πε₀ / G) (1.6 × 10−19 C)2 (1.67 × 10−27 kg) (9.1 × 10−31 kg)
The universal gravitational constant is,
G = 6.67 × 10−11 m3 ⋅ kg−1 ⋅ s−2r3
= (4π × 8.8542 × 10−12 F/m) / (6.67 × 10−11 N m2/kg2) (1.6 × 10−19 C)2 (1.67 × 10−27 kg) (9.1 × 10−31 kg)r
= 5.3 × 10−11 m, approximately.
#SPJ11
Learn more about constant gravitational force:
https://brainly.com/question/11359658
A battery contains two metals that have different tendencies to attract electrons. If one is lithium with an electron affinity of −3.05, and the other is zinc with an electron affinity of −0.76, describe how the electrons will flow. Then, describe how you could make this an even stronger battery.
Answers
This battery could be made stronger when we make lithium the anode and make zinc the cathode.
What is the electron affinity?We know that the term electron affinity has to do with the fact the a specie is able to attract electrons. Hence, the specie that can be able to attract electrons is said to be have a greater electron affinity.
If we look at the order of the reactivity of the metals, we can see that the lithium has more tendency to exist as a positive ion as such the electron affinity of the lithium atom is very negative and it does not attract electrons.
Learn more about electron affinity:https://brainly.com/question/13646318
#SPJ1
A Textbook of
Information
Technology
(Subject code 402)
What is Information technology??
Please don't post invalid answer..!!
Answers
Explanation:
Information Technology CBSE Code 402 for class IX and X is based on National Skills Qualifications Framework. The book is designed and structured to help learner acquire the knowledge and skills regarding conputers, office productivity tools and internet necessary in real life competitive worlf
A team of scientists claim that they have discovered a new experimental
method for determining percent composition. Which of the following is
necessary for the claim to be considered valid?
A. The method must support the law of conservation of mass.
B. The percent compositions for any two compounds made from the
same elements must be the same.
C. Each atom must contribute the same mass to the compound.
D. All scientists using the new method must get the same results.
Answers
Answer:
Option C
Explanation:
The new method must support the law of definite proportions which means that if one mole of compound is distributed percentage wise then the sum of % share of each element must be equal to one mole of compound and this percentage distribution always remains the same in all conditions
Hence, option C is correct
Two imbalances that are related are and hypochloremia because additional Cl-must be excreted to the kidney tubules to buffer the high concentrations of H+ in the tubules. hypokalemia hyperkalemia cations Following hemorrhage can also cause alkalosis because through the renin-angiotensin- aldosterone system Na+ reabsorption is increased causing a larger of H+ into tubular fluid hypercalcemia Systemic acidosis can cause due to the high levels of H+ forcing greater binding of ECF calcium to anions alkalosis
Answers
Two imbalances that are related are hypoglycemia and alkaloids. Hypoglycemia refers to low levels of chloride in the blood, while alkaloids refers to a pH imbalance that leads to a higher than normal alkaline level in the blood.
1. Hypoglycemia and Alkaloids: Hypoglycemia is a condition where there's a low level of chloride (Cl-) in the blood. This can be related to alkaloids, which is a condition where the body's pH is higher than normal. In response to hypoglycemia, the kidney tubules excrete additional Cl- to buffer the high concentrations of H+ in the tubules, which can lead to alkaloids.
2. Alkaloids and Acidosis following Hemorrhage: Hemorrhage can cause alkaloids due to the activation of the rein-angioplasty-testosterone system. This system increases sodium (Na+) re absorption in the kidneys, leading to a higher secretion of H+ ions into the tubular fluid. This can cause an imbalance and potentially lead to alkaloids. Conversely, systemic acidosis, a condition with a lower pH than normal, can occur due to the high levels of H+ ions, forcing a greater binding of extracellular fluid (ECF) calcium to anions, which can also lead to alkaloids.
To know more about hypoglycemia and alkaloids visit:
https://brainly.com/question/4050677
#SPJ11
i need the answers pleaseee! this is acids and bases for chemistry

Answers
Answer:
for pH 13 it = strong based the ph 2 is= weak acid
Explanation:
13p 14n what is this element
Answers
Answer:
it is an isotope of Aluminum
Explanation:
Isotopes are the ones with the same number of protons but different number of neutrons.
The normal Aluminum is 13p+ and 13n. (13+13)