What factors influence the dissolving process for any salt
Answers
Answer:
factors affecting solubility
Temperature. Basically, solubility increases with temperature. ...
Polarity. In most cases solutes dissolve in solvents that have a similar polarity. ...
Pressure. Solid and liquid solutes. ...
Molecular size. ...
Stirring increases the speed of dissolving.
Explanation:
google hope this helps you thank you for helping me
Answer:
Temperature. Basically, solubility increases with temperature. Polarity: In most cases solutes dissolve in solvents that have a similar polarity.Pressure. Solid and liquid solutes. Molecular size. Stirring increases the speed of dissolving.
Related Questions
The half-life for Carbon-14 is 5614 years. An ancient piece of cloth is found to contain ¼ of its original Carbon-14. How old is the cloth? Describe or show in detail how you solved this.
Answers
Answer:
To determine the age of the ancient cloth, we can use the concept of radioactive decay and the half-life of Carbon-14.
Carbon-14 is a radioactive isotope of carbon, which decays over time into nitrogen-14 through beta decay. The half-life of Carbon-14 is 5614 years, which means that after 5614 years, half of the original amount of Carbon-14 in a sample will have decayed.
In this case, the cloth contains only ¼ of its original Carbon-14. This means that three half-lives have passed since the cloth was first created, as each half-life reduces the amount of Carbon-14 by half.
To determine the age of the cloth, we can use the following formula:
N = N0(1/2)^t/T
where N is the current amount of Carbon-14 in the cloth, N0 is the original amount of Carbon-14 in the cloth, t is the time that has passed, and T is the half-life of Carbon-14.
We know that N = ¼ N0, and T = 5614 years. Plugging these values into the formula, we get:
¼ N0 = N0(1/2)^(3/T)
Solving for t, we get:
t = (3/T) * log(2)
Substituting in T = 5614 years, we get:
t = (3/5614) * log(2) ≈ 1,684 years
Therefore, the cloth is approximately 1,684 years old.
In summary, we can use the concept of radioactive decay and the half-life of Carbon-14 to determine the age of the ancient cloth. By knowing the current amount of Carbon-14 in the cloth, we can calculate the time that has passed since it was first created using a simple formula. In this case, the cloth is approximately 1,684 years old.
Which state or states of matter have fixed volume but not a fixed shape?
Answers
Answer:
gas and liquid
Explanation:
Which of the following statements about weathering is false?
Select one:
a. Rocks of different compositions weather at different rates.
b. Heat and heavy rainfall increase the rate of chemical weathering.
c. The presence of soil slows down the weathering of the underlying bedrock.
d. The longer a rock is exposed at the surface, the more weathered it becomes.
Answers
Answer:
c
Explanation:
The presence of soil slows down the weathering of the underlying bedrock. Hence, option C is correct.
What is weathering?Weathering is the breaking down or dissolving of rocks and minerals on Earth's surface.
Heat and heavy rainfall increase the rate of chemical weathering.
Hence, the presence of soil slows down weathering of the underlying bedrock.
Learn more about weathering here:
https://brainly.com/question/14426457
#SPJ2
A compound has the composition 50.7% C, 9.9% H, and 39.4% N. What is the empirical formula?
Answers
Step 1
Empirical formula definition:
In chemistry, the empirical formula of a chemical compound is the simplest whole-number ratio of atoms present in a compound.
-----------------
Step 2
Information provided:
Composition,
50.7 % C
9.9 % H
39.4 % N
-----------------
Step 3
Assuming a 100 grams sample:
50.7 % C => 50.7 g C
9.9 % H => 9.9 g H
39.4 % N => 39.4 g N
-----------------
Step 4
The number of moles of each element is calculated as:
(Please, use your periodic table)
50.7 g C x (1 mole C/12.01 g C) = 4.22 moles C
9.9 g H x (1 mole H/1.008 g H) = 9.82 moles H
39.4 N x (1 mole N/14.00 g N) = 2.81 moles N
-----------------
Step 5
Each number in step 4 will be divided by the smallest one of the 3. It is also needed integer numbers.
4.22 moles C/2.81 moles N = 1.5
9.82 moles H/2.81 moles N = 3.5
2.81 moles N/2.81 moles N = 1
As we need integer numbers, we will multiply all these numbers by 2 as it follows:
C => 1.5 x 2 = 3
H => 3.5 x 2 = 7
N => 1 x 2 = 2
Therefore, the empirical formula will be:
Answer:
\(C_3H_7N_2\)a titration is carried out for 50.00 ml of 0.125m hbr (strong acid) with 0.200m naoh (strong base). a. at what volume does the equivalence point occur? calculate the ph of the solution at the following volumes of naoh added: b. 0.00 ml c. 10.0 ml d. 31.25 ml e. 40.0 ml
Answers
This titration involves adding 50.00 ml of 0.125 M HBr (strong acid) to 0.200 M NaOH. (strong base). The Correct option is A
When the amount of acid added equals the amount of base added, the equivalence point is reached. According to the reaction's stoichiometry, this happens when 31.25 ml of NaOH have been added.
We use the fact that HBr is a strong acid, which implies that it totally dissociates in water to create H+ ions and Br- ions, to determine the pH at different volumes of NaOH supplied. Only the HBr is present at 0.00 ml of NaOH applied, hence the pH may be determined using the initial concentration of H+. We must use the amount of HBr that hasn't been completely neutralised after 10.0 ml of NaOH have been introduced in order to determine the concentration of H+ ions.
Learn more about titration
https://brainly.com/question/31271061
#SPJ4
An element has 2 stable isotopes. One has 13 amu and 1. 07% abundant. The second has 12 amu and 98. 93 abundant. What is the average atomic mass?
Answers
The average atomic mass of this element is 12.0107 amu.Therefore, the average atomic mass of this element is 12.0107 amu.
To find the average atomic mass, we need to take into account the abundance and mass of each isotope. We can use the following formula:
Average atomic mass = (abundance of isotope 1 x mass of isotope 1) + (abundance of isotope 2 x mass of isotope 2)
Plugging in the values given in the question, we get:
Average atomic mass = (0.0107 x 13) + (0.9893 x 12)
Average atomic mass = 0.1391 + 11.8716
Average atomic mass = 12.0107 amu
Therefore, the average atomic mass of this element is 12.0107 amu.
To calculate the average atomic mass of an element with two stable isotopes, you need to multiply the mass of each isotope by its abundance (in decimal form) and then add the results together. Here's the calculation:
Isotope 1: 13 amu * 0.0107 = 0.1391
Isotope 2: 12 amu * 0.9893 = 11.8716
Average atomic mass = 0.1391 + 11.8716 = 12.0107 amu
So, the average atomic mass of this element is 12.0107 amu.
Visit here to learn more about atomic mass : https://brainly.com/question/29117302
#SPJ11
geostrophic winds blow question 7 options: parallel to isobars from low pressure to high pressure from high pressure to low pressure perpendicular to isobars
Answers
Geostrophic winds blow parallel to isobars from low pressure to high pressure from high pressure to low pressure perpendicular to isobars.
This means that they move along lines of equal pressure, rather than moving from areas of high pressure to areas of low pressure or vice versa. Isobars are lines or contours on a weather map that connect areas of equal atmospheric pressure. Geostrophic winds are typically found in the upper atmosphere, where the Coriolis effect is strong enough to balance out the pressure gradient force and cause the winds to move in a straight line. In the lower atmosphere, friction and other factors can cause the winds to deviate from this pattern.
To learn more about Isobars :
https://brainly.com/question/29971663
#SPJ11
What was the time frame?
500 Million Years Ago
900 Million Years Ago
650 Million Years Ago
4.6 to 4 Billion Years Ago
Answers
Answer:
If you are referring to the Precambrian time, the answer is 500 Million Years Ago.
Explanation:
Precambrian time is the time period that started with the formation of Earth and ended about 500 million years ago.
If predators A and B prey upon the same species and predator A is eliminated, the population of predator B will likely __________. a. be eliminated b. increase c. decrease d. remain the same Please select the best answer from the choices provided A B C D
Answers
Answer: B. Increase because there will be more resources for the remaining predators
Answer: B
Explanation: YES
what have you realized about your self after drawing the kite
Answers
what volume of a 0.45 m solution of hcl would just neutralize 210 ml of a solution containing 4.7 g of ba(oh)2 ?
Answers
Volume of the 0.45 m solution of HCl would just to neutralize 210 ml of a solution containing 4.7 g of the Ba(OH)₂ is 0.12 L.
The reaction is given as :
2HCl(aq) + Ba(OH)₂(aq) ---> BaCl₂(aq) + 2H₂O
1 mole of Ba(OH)₂ react with the 2 mole HCl
Molarity of HCl, C1 = 0.45 M
Volume of HCl, V1 =.?
The mass of Ba(OH)₂ = 4.7 g
Moles = mass / molar mass
Moles = 4.7 / 171.34
Moles = 0.027 mol
The moles of HCl = 2 × 0.027
= 0.054 mol
Volume = moles / molarity
Volume = 0.054 / 0.45
Volume = 0.12 L
To learn more about volume here
https://brainly.com/question/17277081
#SPJ4
.The purpose of this buffer system is to:The purpose of this buffer system is to:a) maintain C2H3O2−b) maintain HC2H3O2c) maintain pH
Answers
A buffer system is designed to maintain a specific pH level (option c).
What is the purpose of a buffer system?Buffer systems are essential in biological and chemical processes as they prevent significant changes in pH by resisting alterations in acidity or alkalinity. They consist of a weak acid and its conjugate base (or a weak base and its conjugate acid). When an acid or base is added to the buffer system, the weak acid or base reacts with the added component, minimizing the change in pH.
The buffer system achieves this by absorbing or releasing hydrogen ions (H+) to maintain a relatively constant pH. This ability to regulate pH is crucial for various physiological functions, such as maintaining proper enzyme activity and cellular processes.
Learn more about buffer systems
brainly.com/question/29763040
#SPJ11
14. a student dissolves 0.0100 mole of a weak acid in 0.10 l of water and then titrates the sample with 0.100 m naoh. a total of 40 ml of titrant was required to reach a poh of 9.00. what is the kb of the conjugate base to the weak acid?
Answers
A total of 40 ml of titrant was required to reach a pOH of 9.00.
6.25× 10⁻¹³ is the Kb of the conjugate base to the weak acid.
The identification of the weak acid is the first step in solving this issue. Given that the pOH is 9.00, we may get the pH as follows:
pH + pOH = 14.00
pH = 14.00 - 9.00 = 5.00
We may formulate the dissociation reaction as follows because we know that the weak acid must partially dissociate because the solution is acidic:
A- + H₃O+ = HA + H₂O
where A- is the conjugate base of the weak acid, denoted by the symbol HA.
According to the equation for the weak acid's dissociation, [A-] = [NaOH added] = 0.004 mol/L.
The weak acid's conjugate base is A-, and the following equation relates its Kb (base dissociation constant) to Ka:
Kw = Ka * Kb
where Kw (1.0 ×10⁻¹⁴ at 25°C) is the water ion product constant. Calculating Kb:
Kw = Kb = 1.0 ×10⁻¹⁴/ 0.016 = 6.25× 10⁻¹³
Therefore, 6.25× 10⁻¹³ is the Kb of the conjugate base to the weak acid.
Learn more about Conjugate base
https://brainly.com/question/30225100
#SPJ4
What is the minimum number of grams of CO require
produce 88 grams of CO2?
A) 28 g
B) 88 g
C) 64 g
D) 56 g
Answers
Answer:
The minimum number of grams of CO require
produce 88 grams of CO2 is 64 g.
A 112 gram sample of an unknown metal was heated from 0.0C to 20.0C. The sample absorbed 1004 J of energy. What was the specific heat capacity of this metal? Show your work.
Answers
Answer:
The specific heat of the sample unknown metal is approximately 0.45 J/g °C.
General Formulas and Concepts:
Thermodynamics
Specific Heat Formula: \(\displaystyle q = mc \triangle T\)
m is mass (g)c is specific heat capacity (J/g °C)ΔT is the change in temperatureExplanation:
Step 1: Define
Identify variables.
m = 112 g
ΔT = 20.0 °C
q = 1004 J
Step 2: Solve for c
Substitute in variables [Specific Heat Formula]: \(\displaystyle 1004 \ \text{J} = (112 \ \text{g})(c)(20.0 \ ^{\circ} \text{C})\)Simplify: \(\displaystyle 1004 \ \text{J} = (2240 \ \text{g} \ ^\circ \text{C})c\)Isolate c: \(\displaystyle c = 0.448214 \ \text{J} / \text{g} \ ^\circ \text{C}\)Round [Sig Figs]: \(\displaystyle c \approx 0.45 \ \text{J} / \text{g} \ ^\circ \text{C}\)∴ specific heat capacity c is equal to around 0.45 J/g °C.
---
Topic: AP Chemistry
Unit: Thermodynamics
Nate and Vic are trying to determine the atomic mass of the atom shown below. Nate thinks it has an atomic mass of 11 u, while Vic thinks it has an atomic mass of 6 u. Who is correct and why?
A. Nate is correct. An isotope’s atomic mass is based on the number of protons and neutrons in the nucleus.
B. Vic is correct. An isotope’s atomic mass is based on the number of electrons in the electron cloud.
C. Vic is correct. An isotope’s atomic mass is based on the number of protons in the nucleus.
D. Neither is correct. The atomic mass is based on the number of neutrons in the nucleus. This isotope’s atomic mass is 5 u.
Answers
Answer: A is correct. I don't see the "element shown below," but it isn't needed to answer this question.
Explanation: By definition, the atomic mass is based on the sum of protons (1 AMU each) and neutrons (1 AMU each). Electrons are assigned a value of 0 AMU.
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed. (i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution. Initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction
Answers
Answer:
it is an endothermic reaction
Explanation:
This is because there is a rise in temperature from 20 to 46. this means that the reaction takes in heat from the suuroundings
Calculate the number of grams of platinum in 0.00808 moles Pt.
Answers
to find the mass we multiply the number of moles with the molar mass
0.00808*195=1.5756g
Explain why an organism dies if the respiratory and circulatory system 'paused' for a while.
Answers
Answer:
Without the respiratory system your blood would be useless. The circulatory and respiratory systems work together to circulate blood and oxygen throughout the body
Explanation:
Which question can be used to identify a physical property of an element?
Does a magnet attract the element?
Does rust form when the element is exposed to oxygen?
Do bubbles form when the element is mixed with an acid?
Does the element burn when held over a Bunsen burner flame?
Answers
Answer:
Does a magnet attract the element?
Explanation:
This is because, the attraction of an element by a magnet is a purely physical property. It has to do with the orientation of the electrons in the element. It does not tell us of the composition of the element or how it reacts with other elements. Whereas, the other questions deal with the chemical properties of the element. That is, how it reacts with other elements or substances.
The Chemical Formula For Lead(II) Nitrite Is: Pb(NO2) 2 How Many Oxygen Atoms Are In Each Formula Unit Of Lead (II) Nitrite?
Answers
The number of oxygen atoms in each formula unit of lead nitrite is equal to four.
What is the formula unit?A formula unit can be used to represent the lowest whole number ratio of ions in an ionic compound. The formula mass of an ionic compound is equal to the sum of the atomic masses of the ions in the formula unit.
A formula unit can be described as an empirical formula of any covalent or ionic compound that can be used as an independent entity for stoichiometric calculations.
Given the chemical formula of the Lead(II) nitrite is Pb(NO₂)₂. The number of oxygen atoms in each formula unit is four.
Learn more about formula unit, here:
brainly.com/question/15971114
#SPJ1
What is morning wood?
Answers
Answer:
nocturnal penile tumescene
Explanation:
is a spontaneous erection of penis during sleep or when waking up.
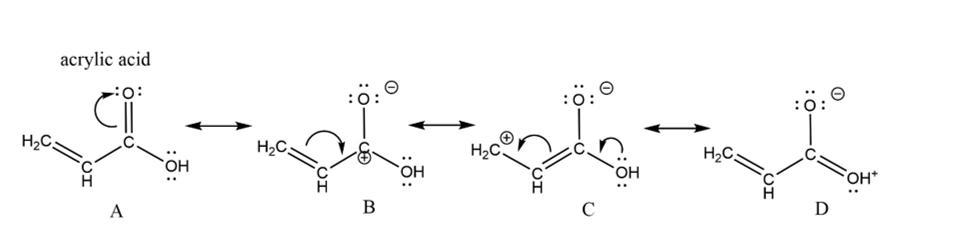
draw curved arrow notation to convert the structure to a resonance structure where all atoms have octets.
Answers
The resonance structures are the set of the lewis structures which describes the delocalization of the electrons in the molecule.
The resonance structures shows the delocalization of the electrons in the molecule. The resonance structures are used to represent the multiple structures of the single polyatomic molecule. The resonance structures have the same number of the electrons, we can not add or subtract the electrons.
All the resonance structures should follow the rules of the writing the lewis structures. The same number of the paired and the unpaired of the electrons should take part in the resonance. The resonance structures are attached below .
To learn more about resonance here
https://brainly.com/question/28734136
#SPJ4
State two characterstics of matter demostred
by
Diffusion
.) Brownian mation
Answers
For Brownian motion, it could be: particles have spaces between them, particles are incredibly small, and that particles are always, continuously moving.
Hope this helps!
How are elements similar in a group/family on the periodic table?
Answers
All the elements in each group have the same number of electrons in their outer orbitals, also known as valence electrons.
What is the molecular geometry of BCl3
a. otriginal planar
b. trigonal pyraminad
c. tetrahedral
d. liner

Answers
What is the molar concentration a a 12 % sodium chloride solution (MW 58.5)
Answers
The molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To determine the molar concentration of a 12% sodium chloride solution, we need to convert the given percentage concentration into molarity.
First, we need to understand that the percentage concentration refers to the mass of the solute (sodium chloride) relative to the total mass of the solution.
In this case, a 12% sodium chloride solution means that there are 12 grams of sodium chloride in 100 grams of the solution.
To convert this into molar concentration, we need to consider the molar mass of sodium chloride, which is 58.5 g/mol.
We can start by calculating the number of moles of sodium chloride in 12 grams:
Moles of sodium chloride = mass of sodium chloride / molar mass of sodium chloride
Moles of sodium chloride = 12 g / 58.5 g/mol = 0.205 moles
Next, we calculate the volume of the solution in liters using the density of the solution. Since the density is not provided, we assume a density of 1 g/mL for simplicity:
Volume of solution = mass of solution / density
Volume of solution = 100 g / 1 g/mL = 100 mL = 0.1 L
Finally, we calculate the molar concentration (Molarity) by dividing the number of moles by the volume in liters:
Molar concentration = moles of solute / volume of solution
Molar concentration = 0.205 moles / 0.1 L = 2.05 M
Therefore, the molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To learn more about molarity click here: brainly.com/question/31545539
#SPJ11
Which type of interaction stabilizes the alpha (α) helix and the beta (β) pleated sheet structures of proteins?
Answers
The type of interaction that stabilizes the alpha (α) helix and the beta (β) pleated sheet structures of proteins is called hydrogen bonding.
Hydrogen holding is an extraordinary sort of dipole fascination between particles, not a covalent cling to a hydrogen iota. It results from the appealing power between a hydrogen particle covalently clung to an extremely electronegative molecule like a N, O, or F iota and another exceptionally electronegative iota.
Hydrogen bonds can occur in any molecule that has a hydrogen atom directly attached to an oxygen or nitrogen. When hydrogen is bonded to fluorine, hydrogen bonds also occur, but other molecules lack the HF group.
Know more about hydrogen bonding:
https://brainly.com/question/31139478
#SPJ11
When cations and anions meet, they. A. repel. B. form ionic bonds. C. form covalent bonds. D. form individual molecules
Answers
When cations and anions meet, they B. form ionic bonds.
Why cations and anions can form an ionic bond?This is because cations are positively charged ions and anions are negatively charged ions. When they come together, they form an ionic bond, which is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions.
This is different from a covalent bond, which is formed when atoms share electrons to form molecules.
Therefore, the correct answer is B. form ionic bonds.
Learn more about ionic bonds here https://brainly.com/question/957239
#SPJ11
what will happen when the rates of evaporation and condensation are equal