what energy, in megaelectron volts, is released in this decay? the mass of the daughter nuclide is 231.036298 u.
Answers
The daughter nuclide's mass is 231.036298 u, and the energy released inside the alpha decay is measured in megaelectron volts [Q = Delta mc2].
How do you define a nuclide?Nuclide, also known as nuclear species, is an atom species that is identified by the amount of protons, neutrons, and also the energy level of a nucleus. As a result, a nuclide is identified by its quantity (A) and atomic number (Z).
What is a typical nuclide?Carbon-12 is the nuclide that is used as the reference point to assess how atomic masses compare to one another. Calculated at 1/12 the mass of an atom of carbon-12, the comparative atomic mass unit. Despite having similar masses in this system, protons and neutrons have slightly different masses.
To know more about nuclide visit:
https://brainly.com/question/9826865
#SPJ4
Related Questions
The molar mass of ammonium acetate is 77.083 g/mol. A student uses 0.100 mol of ammonium acetate in a chemical reaction. The
student claims that the reaction uses (0.100 mol) (77.083 g/mol) = 7.71 g of ammonium acetate, which has
(7.71) (6.022 x 1023) = 4.64 x 1024 molecules.
In one to two sentences, explain the mistake that the student made and determine the correct number of molecules of ammonium
acetate used in the reaction.
Answers
The student's claim of 4.64 × 10^24 molecules is incorrect, and the correct number of molecules of ammonium acetate used in the reaction is 6.022 × 10^22 molecules.
The mistake the student made is assuming that the molar mass of ammonium acetate directly corresponds to the number of molecules. However, the molar mass of a substance represents the mass of one mole of that substance, not the number of molecules.
To determine the correct number of molecules of ammonium acetate used in the reaction, we need to use Avogadro's number, which relates the number of particles (atoms, molecules, etc.) in one mole of a substance.
Avogadro's number is approximately 6.022 × 10^23 particles/mol. Given that the student used 0.100 mol of ammonium acetate, we can calculate the correct number of molecules by multiplying the number of moles by Avogadro's number:
Number of molecules = (0.100 mol) × (6.022 × 10^23 molecules/mol)
Performing the calculation, we find that the correct number of molecules of ammonium acetate used in the reaction is 6.022 × 10^22 molecules.
For more such questions on ammonium acetate visit:
https://brainly.com/question/29570260
#SPJ8
How many kilograms does a 1.60 ton elaphant weigh
Answers
Answer:
1451.496
Explanation:
A unit of weight equal to 1,000 kilograms, or approximately 2,204.6 pounds
tons =
kg
______
1000.0
What is the name of Sn3(PO4)4 under the Stock system of nomenclature?
Answers
Answer:
Tin (IV) Phosphate
Explanation:
"Stock nomenclature for inorganic compounds is a widely used system of chemical nomenclature developed by the German chemist Alfred Stock and first published in 1919. In the "Stock system", the oxidation states of some or all of the elements in a compound are indicated in parentheses by Roman numerals."
Rank the given compounds based on their relative Brensted acidities. strongest Bronsted acid,weakest Bronsted acid H-CH_3, H-OH, H-I, H-F, H-NH_2
Answers
The compounds ranked based on their relative Bronsted acidities from strongest to weakest are as follows:
1. H-I (Hydrogen iodide)
2. H-CH3 (Methyl radical)
3. H-OH (Hydroxide ion)
4. H-NH2 (Ammonia)
5. H-F (Hydrogen fluoride)
Bronsted acidities can be determined by analyzing the stability of the corresponding conjugate bases. A stronger acid will have a more stable conjugate base. Here is the explanation for the ranking:
1. H-I: Hydrogen iodide (HI) is a strong acid because iodide ion (I-) is a stable conjugate base. Iodide ion is large and can effectively disperse negative charge, leading to stability.
2. H-CH3: Methyl radical (CH3) is weaker than HI but stronger than the remaining compounds. It is a stable radical and has resonance structures that stabilize its conjugate base.
3. H-OH: Hydroxide ion (OH-) is less acidic than HI and CH3. It forms a stable conjugate base, but it is not as stable as iodide ion or the methyl radical.
4. H-NH2: Ammonia (NH3) is weaker than the previous compounds. The lone pair on the nitrogen atom can be donated to accept a proton, making NH2- a relatively unstable conjugate base.
5. H-F: Hydrogen fluoride (HF) is the weakest acid among the given compounds. The fluoride ion (F-) is a relatively strong base, and its conjugate acid, HF, is a weaker acid compared to the others.
The ranking of the given compounds based on their relative Bronsted acidities, from strongest to weakest, is H-I, H-CH3, H-OH, H-NH2, and H-F. This ranking is determined by analyzing the stability of their respective conjugate bases, with stronger acids having more stable conjugate bases.
Learn more about the Bronsted acidities visit:
https://brainly.com/question/15516010
#SPJ11
How does an electric generator work?
Answers
Answer:
A conductor coil (a copper coil tightly wound onto a metal core) is rotated rapidly between the poles of a horseshoe type magnet. ... The magnetic field will interfere with the electrons in the conductor to induce a flow of electric current inside it.
A student wants to determine if a
sample of tap water contains Mg+2.
Which of the following pieces of
information are not needed to do a
titration ?
Answers
Answer:
The Density of Magnesium.
Explanation:
can sb helppppppppppppp

Answers
Explanation:
Copper does not react with HCl to give H2 gas.
Which of the following is a true difference between protons and neutrons?
a
the have different masses
b
they are in different locations in the atom
c
the proton identifies the atom and the neutrons does not
d
all of the choices are true
Answers
Answer:
D
Explanation:
6. How is Avogadro’s number used to convert moles into particles?
Answers
Explanation:
Avogadro's number is a very important relationship to remember: 1 mole = 6.022×1023 6.022 × 10 23 atoms, molecules, protons, etc. To convert from moles to atoms, multiply the molar amount by Avogadro's number.
I need help with question 4!!!!!!!
*Please choose correctly*
Thank youuuu
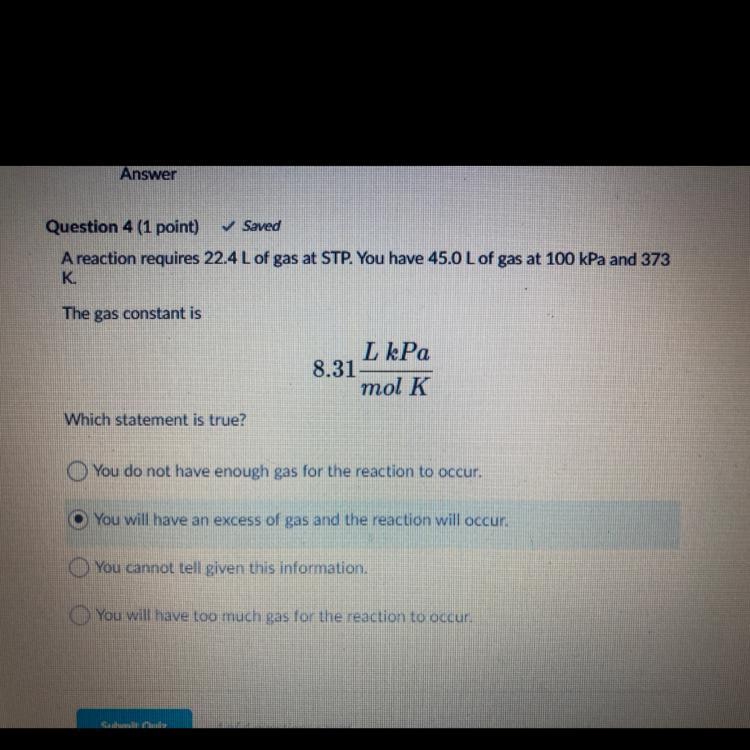
Answers
Answer:
hshzjndbejkxn. znkskakannamzm msk
What observation did Rutherford make from his gold-foil experiment which
ultimately supported his theory?
Answers
Answer:
A piece of gold foil was hit with alpha particles, which have a positive charge. Most alpha particles went right through. This showed that the gold atoms were mostly empty space. Some particles had their paths bent at large angles. A few even bounced backward. The only way this would happen was if the atom had a small, heavy region of positive charge inside it.
What volume of air at room conditions (20c , 760 torr) would be required to combust a full lighter of butane
Answers
The amount of air required to combust a full lighter of butane can be calculated using the stoichiometry of the combustion reaction. The complete combustion of butane (C4H10) can be represented by the following equation:
C4H10 + 6O2 -> 4CO2 + 5H2O
From this equation, we can see that for every mole of butane combusted, 6 moles of oxygen are required. To determine the volume of air required, we need to know the concentration of oxygen in air.
Air is composed of approximately 21% oxygen and 79% nitrogen, and since the pressure of air is 760 torr, the partial pressure of oxygen is:
0.21 * 760 torr = 160 torr
learn more about butane here:
https://brainly.com/question/14818671
#SPJ4
How many significant figures are there in
9.710 x 10-4
Answers
Answer:
91.3
2 Significant figures
Answer:
The values have 2 significant numbers ╮( ̄ω ̄;)╭
Give the formula for the compound formed between the following ions:
Aluminum and hydroxide
2 pc
Answers
Answer:
Al(OH)3
Explanation:
-Aluminum hydroxide is an antacid. Aluminum hydroxide is used to treat heartburn, upset stomach, sour stomach, or acid indigestion. Aluminum hydroxide is also used to reduce phosphate levels in people with certain kidney conditions. Aluminum hydroxide may also be used for purposes not listed in this medication guide.
-Aluminium hydroxide is an inorganic basic compound used as an intermediary in organic synthesis and as an additive in pharmaceutical and fine chemical industries. Formula and structure: The aluminum hydroxide chemical formula is Al(OH)3 and its molar mass is 78.00 g mol-1.
(science)
HURRY 5 MINUTES
What kind of drug is nicotine? a. anabolic b. depressant c. stimulant d. inhalant
Answers
Answer:
Nicotine is classified as a stimulant drug.
Three elements with consecutive atomic numbers are known to be isoelectronic. The largest and smallest have atomic radii of 1.84 and 1.36 angstroms. Estimate and explain the radius of the third element.
Answers
The radius of the third element to be between 1.36 and 1.84 angstroms. The specific value would depend on the exact nature of the elements and their position in the periodic table.
Isoelectronic elements have the same number of electrons, which means they have similar electronic configurations and therefore similar atomic radii. Since the largest and smallest elements in the series have atomic radii of 1.84 and 1.36 angstroms respectively, we can assume that the third element, which is isoelectronic with them, will have a similar atomic radius.
By observing the trend between the atomic radii of consecutive elements in the periodic table, we can estimate the radius of the third element. As we move across a period from left to right, the atomic radius generally decreases due to increasing effective nuclear charge.
Therefore, the atomic radius of the third element is likely to be smaller than that of the largest element but larger than that of the smallest element.
learn more about atomic radii :
https://brainly.com/question/29482631
#SPJ4
Genetic diversity is important because the more genes are in a population , the more likely that one of those genes will prove ______ in the face of threats such as climate change or a new disease.
Answers
Answer:
higher endurance & adaptability
Explanation:
Genetic Diversity is diversity within species, community.
Genetic Diversity is important as higher genetic variations in population imply that - some or all of their genes have will prove to have higher endurance & adaptability in case of environment aspect (like climate) change or any adversity (new disease).
Can someone help me!!!!!

Answers
Answer:
Can u somehow make it bigger please?
Explanation:
what is the torsional angle between c-ch 3 bonds in the totally eclipsed conformation of butane?
Answers
The torsional angle between c-ch 3 bonds in the totally eclipsed conformation of butane is 0°.Explanation:In the totally eclipsed conformation of butane,
there is a high degree of torsional strain. The eclipsed conformation has the highest energy level of all butane conformations because of the steric hindrance caused by the overlapping atoms. Atoms that overlap produce strain energy, which raises the energy of the molecule. In the butane molecule,
the maximum torsional angle for the C-C bond is 120 degrees, and the minimum torsional angle is 60 degrees.Therefore, in the totally eclipsed conformation, the C-C bond angle is 0° because the two methyl groups are eclipsed.
TO know more about that torsional visit:
https://brainly.com/question/31838400
#SPJ11
A skydiver is in a plane flying at a constant velocity. If the skydiver is trying to land on a
target on the ground, should he jump from the plane when he is directly over the target?
Why or why not?
Answers
Answer:
No,it is not possible to jump directly because the upper body parts are In motion but legparts directly comes in rest due to unbalanced inertia he may get hurt so..
When Acid Rain falls on limestone, the rock is eroded and carbon dioxide is produced.
Write a balanced equation for the reaction between calcium carbonate and nitric acid.
Answers
The formula of calcium carbonate is CaCO3
The formula of nitric acid is HNO3.
When put together:
CaCO3 + HNO3 = Ca(NO3)2 + CO2 + H2O
The balanced equation:
CaCO3 + 2HNO3 = Ca(NO3)2 + CO2 + H2O
discuss the molar mass and identity of your unknown based on the freezing point depression. are you confident in the identity of the unknown referencing % error
Answers
Molar mass is ratio of mass and the amount of same substance whose mass we have taken.Yes,I am confident in the identity of the unknown referencing % error.
In science, the molar mass (M) of a chemical compound is characterized as the proportion between the mass and how much substance (estimated in moles) of any example of said compound. The molar mass is a mass, not sub-atomic, property of a substance. The molar mass is a normal of many occurrences of the compound, which frequently shift in mass because of the presence of isotopes. Most usually, the molar mass is figured from the standard nuclear loads and is in this manner an earthly normal and a component of the overall wealth of the isotopes of the constituent particles on The planet. The molar mass is fitting for changing over between the mass of a substance and how much a substance for mass amounts.
Freezing point depression is a drop in the base temperature at which a substance freezes, caused when a more modest measure of another, non-unpredictable substance is added. Models incorporate adding salt into water (utilized in frozen yogurt creators and for de-icing streets), liquor in water, ethylene or propylene glycol in water (utilized in radiator fluid in vehicles), adding copper to liquid silver (used to make bind that streams at a lower temperature than the silver pieces being joined), or the blending of two solids like pollutions into a finely powdered drug.
To know more about molar mass,viist here:
https://brainly.com/question/22997914
#SPJ4
. What’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0%
oxygen?
Answers
Answer:
3 moles of carbon, 3 moles of hydrogen and 1 mole of oxygen
Why do we keep the volume of the rinses small in the recrystallization procedure.
Answers
In recrystallization, the use of a small rinse volume helps to retain more of the desired product and minimize loss during the purification process.
Recrystallization is a method used to purify solid compounds. It involves dissolving the compound in a suitable solvent and then slowly cooling the solution.
As the solution cools, the solute (compound) crystallizes out, leaving behind impurities in the solvent.
To prevent the dissolution of the product in the solvent, the volume of the rinses in the recrystallization process is kept small.
If the rinses have a large volume, there is a risk of losing some of the desired product along with the impurities.
By minimizing the volume of the rinses, the loss of product can be reduced, improving the overall yield and purity of the final crystallized compound.
In recrystallization, the use of a small rinse volume helps to retain more of the desired product and minimize loss during the purification process.
This ensures a higher-quality final product with reduced impurities.
To know more about Recrystallization procedure here: https://brainly.com/question/32814473
#SPJ11
The equilibrium SO₂Cl₂ (g) --> SO₂ (g) + Cl₂ (g) is attained at 25 °C in a closed container.
When the concentration of Cl₂ is increased keeping the temperature constant, which of the following statements are correct?
a) Concentration of SO₂ is increased
b) Concentration of SO₂Cl2 is decreased
c) Concentration of SO₂ is decreased
d) None of the above
Answers
Explanation:The equilibrium expression for the reaction can be written as follows:
Kc = [SO2Cl2] / [SO2] [Cl2]
At equilibrium, the concentration of reactants and products remain constant. When the concentration of Cl2 is increased while keeping the temperature constant, the reaction will shift to the right in order to consume the excess Cl2. This will result in an increase in the concentration of SO2 and a decrease in the concentration of SO2Cl2.
So, the correct statement is b) Concentration of SO2Cl2 is decreased.
To learn more about equilibrium,
https://www.toppr.com/ask/question/the-equilibrium-s-o-2-cl-2/#acceptedAnswer
what is a complex permanent tissue? mention the major functions of this tissue .
Answers
Answer:
Complex permanent tissue is defined as a collection of structurally dissimilar cells performing a common function or set of functions.
its functions are:
1.help in the transportation of organic material, water, and minerals up and down the plants.
2.it helps in transportation of food from leaves to other parts of plants.
have a great dayyyyyy
help me i put max points

Answers
Answer:
D. Catastrophism
Explanation:
Answer:
Catastrophism is the answer :p lol
Explanation:
What is the temperature -71 oC expressed in Kelvin?
Group of answer choices
Answers
Answer: 202.15
Explanation:
-71°C + 273.15
= 202.15K
What is the volume of 0.05 mol of neon gas
Answers
Answer:
1.12L
Explanation:
multiply 0.05 ×22.4
what is the name of this atom?
A.) Beryllium
B.) Boron
C.) Fluorine
D.) Helium

Answers
Answer:
Explanation:
A .