Answers
Answer:
carbon oxygen
Explanation:
Related Questions
Which of the following factors affects the amount of heat absorbed by a substance?
A. Boiling point of the substance
B. Specific heat capacity of the substance
C. Melting point of the substance
D. Shape of the substance
Answers
Answer:
The correct answer is B, "Specific heat capacity of the substance."
Explanation:
I just took the test. I hope this helps!
Heat absorbed changes the bonds of the molecules or the compounds to alter their state. The specific heat capacity affects the amount of heat absorbed. Thus, option B is correct.
What is specific heat capacity?Specific heat capacity is defined as the amount of heat or energy absorbed by the kilogram of the solid reactant when the system experiences a rise in temperature. It is measured in J/(kg K) or J/(kg °C).
The specific heat capacity, the mass of the substance, temperature, and the phase of the substance affects the absorption of the heat from the surrounding. The specific heat affects absorption as it tells about the substance's ability to take the heat energy.
Therefore, specific heat capacity affects heat absorption.
Learn more about specific heat capacity here:
https://brainly.com/question/2530523
#SPJ5
What is the atomic mass of an electron?
Answers
The atomic mass of an electron is 0.00054858 amu (atomic mass units). An electron is a subatomic particle that has a negative electrical charge.
Electrons are the smallest of all particles in an atom and make up most of the atom's mass. Electrons are found in the electron cloud, which is the region around the nucleus of an atom. Electrons are very small and have no known structure or size. They move around the nucleus in a wave-like pattern and can be considered to act like particles and waves at the same time. Electrons are responsible for many of the chemical and physical properties of atoms, including electrical conductivity, magnetism, and light emission.
To learn more about atomic mass click here https://brainly.com/question/5661976
#SPJ4
all monosaccharides and disaccharides dissolve in water. why is this?
Answers
Monosaccharides and disaccharides dissolve in water because they have hydrophilic groups, which form hydrogen bonds with water molecules.
This allows the sugar molecules to be surrounded by water molecules, and therefore dissolve in water.Water is a polar solvent and therefore interacts well with other polar solutes. Hydrophilic groups found in both monosaccharides and disaccharides such as hydroxyl groups and carbonyl groups (C=O) are soluble in water, making these sugar molecules soluble in water.
Because the solubility of any substance is dependent on the polarity of the solvent and solute, it is the presence of the hydrophilic groups found in sugars that allows them to dissolve in water. This allows the sugar molecules to be surrounded by water molecules, and therefore dissolve in water.Water is a polar solvent and therefore interacts well with other polar solutes.
To know more about Monosaccharides visit :
https://brainly.com/question/29679417
#SPJ11
3 point different between reactant and product
Answers
Answer:
reactants
The substance(s) to the left of the arrow in a chemical equation are called reactants. A reactant is a substance that is present at the start of a chemical reaction
Explanation:
product_The substance(s) to the right of the arrow are called products . A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product.
There is a standard way of writing chemical equations. The reactants are all written on the left-hand side of the equation, with the products on the right-hand side. An arrow points from the reactants to the products to indicate the direction of the reaction:
reactants → products
Reactants
1. The substances used as starting materials and which react with one another are reactants.
2. Example: In this reaction Mg and O2 are reactants.
Products
1. The substances which are formed as a result of reaction are products.
2. Example: In this reaction MgO is a product.
Which of the following is not a property of a gas ?
A. assumes the shape of its container.
B. has no definite volume.
C. has a definite shape.
D. easily compressible.
Answers
Which of the following statements on HPLC modes is true? A. Increasing the polarity of the mobile phase decreases the elution time of polar compounds in normal-phase HPLC B. A non-polar stationary phase is used in normal-phase HPLC C. Compounds have a lower attraction to the mobile phase than to the stationary phase in displacement development D. A polar stationary phase is used in reversed-phase HPLC E. More polar compounds elute first in normal-phase HPLC
Answers
The following statements on HPLC modes are true is more polar compounds elute first in normal-phase HPLC (Option E).
The liquid chromatography (HPLC) is a technique in analytical chemistry employed for the separation, identification, and quantification of elements. It is considered a highly sensitive method, and it works by separating the components in a mixture with the assistance of a solvent under high pressure.
There are two modes of HPLC: Reversed-Phase HPLC (RP-HPLC) and Normal-Phase HPLC (NP-HPLC). In RP-HPLC, a nonpolar stationary phase, such as C18, is used, and polar solvents, such as water, are used as mobile phases. Polar stationary phases, such as silica gel, are used in NP-HPLC, while nonpolar solvents, such as hexane, are used as mobile phases.
More polar compounds have a greater affinity for the polar stationary phase than less polar compounds, which have a higher affinity for the nonpolar mobile phase in NP-HPLC. As a result, less polar compounds elute first in normal-phase HPLC.
Thus, the correct option is E.
Learn more about HPLC: https://brainly.com/question/13490391
#SPJ11
Which best describes Lenny’s error?
In Step 1, he should have found the square root of 400 J instead of squaring 8 m/s.
In Step 1, he should have multiplied 8 m/s by 2 instead of squaring it.
In Step 2, he should have divided 64 m2/s2 by 400 J instead of dividing 400 J by 64 m2/s2.
In Step 3, he should have multiplied 6.25 J per m2/s2 by 2 instead of dividing it by 2.
Answers
In Step 3, he should have multiplied 6.25 J per m2/s2 by 2 instead of dividing it by 2 best describes Lenny’s error. Therefore, option D is correct.
What is kinetic energy ?The term kinetic energy is defined as the energy that it possesses due to its motion. It is also defined as the work needed to accelerate a body of a given mass from rest to its stated velocity.
The object contain an kinetic energy,
Given Mass of an object = 400 J
Energy moving at a velocity = 8 m/s.
Ek = 1/2 mv²
= 400J = 1/2 × m 64
= 400J/ 64= 1/2 m
= 6.25 × 2
= 12.25kg
So, In the step 3 ,he should have multiplied 6.25 J per m²/s² by 2 instead of dividing it by 2.
Thus, option D is correct.
To learn more about the kinetic energy, follow the link;
https://brainly.com/question/15764612
#SPJ1
Your question is incomplete, most probably your question was
Lenny wrote the steps he used to find the mass of an object with 400 J of energy moving at a velocity of 8 m/s.
1. Find the square of 8 m/s, which is 64 m2/s2.
2. Divide kinetic energy, 400 J, by 64 m2/s2, which is 6.25 J per m2/s2.
3. Divide 6.25 J per m2/s2 by 2 to get a mass of 3.125 kg.
Which best describes Lenny’s error?
A. In Step 1, he should have found the square root of 400 J instead of squaring 8 m/s.
B. In Step 1, he should have multiplied 8 m/s by 2 instead of squaring it.
C. In Step 2, he should have divided 64 m2/s2 by 400 J instead of dividing 400 J by 64 D. m2/s2.
In Step 3, he should have multiplied 6.25 J per m2/s2 by 2 instead of dividing it by 2.
The second-order rate constant for the decomposition of NO2 (to NO and O2) at 573 K is 0.54 M-1 s-1. Calculate the time for an initial NO2 concentration of 0.20 mol/L to decrease to a) one-half; b) one-sixteenth; c) one-ninth of its initial concentration.
Answers
The rate of second order reaction depends on the change in the concentration of the reactants.
Second order reaction:A second order reaction is one in which the rate of reaction depends on the concentration of reactants.
For a second order reaction;
1/[A] = kt + 1/[A]o
When concentration deceresases to one-half;
1/0.1 = 0.54t + 1/0.20
1/0.1 - 1/0.20 = 0.54t
10 - 5/0.54 = t
t = 9.3 s
When concentration deceresases to one-sixteenth;
1/0.0125 = 0.54t + 1/0.20
1/0.0125 - 1/0.20 = 0.54t
80 - 5 = 0.54t
t = 80 - 5/0.54
t = 149 s
When concentration deceresases to one-ninth;
1/0.022 = 0.54t + 1/0.20
1/0.022 - 1/0.20 = 0.54t
45 - 5 = 0.54t
t = 45 - 5/0.54
t = 74 s
Learn more about second order reaction: https://brainly.com/question/12446045
Please help with this!!!

Answers
(a) \(CH_{3} OH\): 3 moles
(b) \(CH_{2} =CHCH_{3}\) : 6 moles
(c) \(CH_{3} OCH_{3}\) : 5 moles
(d) CH=CH: 3 moles
The number of moles of oxygen required for the complete combustion of different compounds can be calculated by writing the balanced chemical equation for the combustion reaction.
For example, the combustion of methanol (\(CH_{3} OH\)) requires 3 moles of oxygen for every 2 moles of \(CH_{3} OH\). Similarly, the combustion of 1-butene (\(CH_{2} =CHCH_{3}\)) requires 6 moles of oxygen for every 1 mole of \(CH_{2} =CHCH_{3}\). The combustion of dimethyl ether (\(CH_{3} OCH_{3}\)) requires 5 moles of oxygen for every 2 moles of \(CH_{3} OCH_{3}\).
The combustion of ethene (\(CH_{2}=CH_{2}\)) requires 3 moles of oxygen for every 1 mole of CH=CH. Knowing the required amount of oxygen is important to calculate the stoichiometry of a reaction and the efficiency of combustion reactions.
To learn more about combustion, refer:
https://brainly.com/question/32079208
#SPJ1
Which of the following is FALSE about bisphenol A (BPA)?
A) BPA is found in canned food and plastic products.
B) BPA was banned by the FDA in 2009 due to safety concerns.
C)BPA has been linked to breast cancer and prostate cancer.
D)BPA is a synthetic hormone.
Answers
The FALSE statement about bisphenol A (BPA) is B) BPA was banned by the FDA in 2009 due to safety concerns.
What is bisphenol A?
Bisphenol A (BPA) is a synthetic compound that is utilized in the production of plastic materials such as polycarbonates, resins, and epoxy resins.
It is employed to make water bottles, baby bottles, and other items that we use on a daily basis.
However, studies have indicated that exposure to BPA can lead to health issues.
In particular, the chemical has been linked to breast cancer, prostate cancer, and other health issues.
The false statement about bisphenol A (BPA) is B) BPA was banned by the FDA in 2009 due to safety concerns.
Explanation:The other statements about bisphenol A (BPA) are true.
It is commonly found in canned foods and plastic products, as well as other household items.
Studies have linked exposure to BPA to several health concerns, including breast cancer and prostate cancer. Although the FDA has taken steps to limit the use of BPA in certain products, such as baby bottles and sippy cups, it has not been banned entirely due to concerns about its impact on the economy and industry.
learn more about bisphenol A on
https://brainly.com/question/1705465
#SPJ11
Which process is an example of a chemical change? drying clothes in the dryer heating a cup of tea chopping a tree activating a glow stick
Answers
Activating a glow stick is the example of a chemical change, as snapping one will cause a chemical reaction, causing the glow. All of the other options are physical changes because nothing new is formed. Hope this helps!
Scenario: The effectiveness of various metals in preventing rusting of iron.
Several weeks after Allen conducted a classroom experiment on the effectiveness of various metals in releasing hydrogen gas from hydrochloric acid, he read that the gas company was burying sheets of magnesium next to iron pipelines in order to prevent rusting. Allen wondered if other active metals would also be effective in preventing rust.
To investigate, he placed each of the following into a separate test tube containing water: one iron nail; one iron nail wrapped with an aluminum strip; one iron nail wrapped with a magnesium strip; and one iron nail wrapped in a lead strip. He used the same amounts of water from the same source, equal amounts of the metal wraps and the same type of iron nails. At the end of five days, he described the amounts of rusting either as small, moderate or large. He also recorded the color of the water.
Allen predicted that this the lead and aluminum would be less effective than the aluminum. Question 15 pts
What is the primary question under investigation in this scenario?
Group of answer choices
Will other metals rust as quickly as iron?
Will metals other iron prevent the rusting of iron?
Does magnesium dissolve as quickly as aluminum or lead?
Which type of iron nails are best for building with wood?
Question 25 pts
What is the analysts' hypothesis concerning the experiment?
Group of answer choices
The size of the test tubes will affect the rate of rusting more than the type of metal wrapped around the nail.
The rate of rusting will be unaffected by any of the other metals.
The magnesium will reduce the rate at which iron rusts more than the aluminum or lead.
The magnesium will reduce the rate at which iron rusts less than the aluminum or lead. Question 35 pts
What is the independent variable in this scenario?
Group of answer choices
the amount of time
the temperature
the amount of hydrochloric acid
the type of metal wrapped around the nail
Question 45 pts
What is the dependent variable in this scenario?
Group of answer choices
what type of metal is used
how much each nail rusts
how hard it is to wrap the test metal around the nail
the color of the waterQuestion 55 pts
What factors are kept constant in this scenario? Select all constants.
Group of answer choices
the amount of water
the source of the water
the type of test tube
the amount of metal wrapped around the nail
Answers
What is the pH of salt solution due to hydrolysis of salt of weak acid and strong base?
Answers
The pH of a salt solution due to hydrolysis of a salt of a weak acid and a strong base will depend on the nature of the ions involved and their interactions with water.
If the salt is derived from a weak acid and a strong base, such as sodium acetate (NaCH3COO), the acetate ion (CH3COO-) is derived from the weak acid (acetic acid, CH3COOH) and the sodium ion (Na+) is derived from the strong base (sodium hydroxide, NaOH). In this case, the acetate ion can react with water in a hydrolysis reaction:
CH3COO- + H2O ⇌ CH3COOH + OH-
The hydrolysis of acetate ion produces hydroxide ions (OH-), resulting in an alkaline solution. As a result, the pH of the salt solution will be greater than 7, indicating alkalinity.
On the other hand, if the salt is derived from a strong acid and a weak base, such as ammonium chloride (NH4Cl), the ammonium ion (NH4+) is derived from the weak base (ammonia, NH3) and the chloride ion (Cl-) is derived from the strong acid (hydrochloric acid, HCl). In this case, the ammonium ion can react with water in a hydrolysis reaction:
NH4+ + H2O ⇌ NH3 + H3O+
The hydrolysis of ammonium ion produces hydronium ions (H3O+), resulting in an acidic solution. Therefore, the pH of the salt solution will be less than 7, indicating acidity.
For more such questions on salt solution visit:
https://brainly.com/question/23269908
#SPJ8
How many grams of sodium hydroxide are required to prepare a 200 ml solution of a 10% (weight per volume) solution? (Atomic weights: Na = 23; 0 = 16; H = 1)
Answers
Given that we want to prepare a 10% solution of sodium hydroxide in 200 mL, we need to calculate the mass of sodium hydroxide required to make this solution.
We can use the following formula to calculate the mass of solute required to make a given volume and concentration of solution:
mass of solute = volume of solution x concentration of solution x density of soluteFirst, let's calculate the density of sodium hydroxide.The density of solid NaOH is 2.13 g/mL. So, the density of sodium hydroxide solution is:
Density = 2.13 g/mLNow, let's substitute the given values into the formula to calculate the mass of sodium hydroxide required:mass of solute = 200 mL x 0.10 x 2.13 g/mL= 4.26 gTherefore, 4.26 grams of sodium hydroxide are required to prepare a 200 mL solution of a 10% (weight per volume) solution.About Sodium hydroxideSodium hydroxide, also known as lye and caustic soda or caustic soda, is an inorganic compound with the chemical formula NaOH. This compound is an ionic compound in the form of a white solid composed of the sodium cation Na⁺ and the hydroxide anion OH⁻. Sodium hydroxide is a building block that can also be found in detergents and oil stain removers. We use it to make products clean better by influencing the formula molecules, so they work better together.
Learn More About Sodium hydroxide at https://brainly.com/question/25866669
#SPJ11
How many protons does Royalty, R-13, have?
Royalty is element #6.
O 13
07
06
O 19
Answers
please lmk if i was right:))
The specific heat capacity of silver is 0.24 J/g °C. How many joules of energy are needed
to warm 0.500 g of silver from 25.0°C to 27.5°C?
Answers
Answer:
0.3 J
Explanation:
The equation for heat capacity is Q = mcΔT where Q is the heat, m is the mass of the substance, c is the specific heat capacity of the substance and delta T is the change in temperature. Plugging those values into the equation, we have Q = (.500)(0.24)(27.5-25) = 0.3
The pH of a weak-acid solution is calculated using systematic treatment; that means writing down same number of independent equations as there are unknown concentrations. Which of the following is NOT an equation that is needed to solve for pH? [H][4] L. K= THA CHA total = [HA] + [A") [H] = [A-] + [OH-] [HA]OH IV. Ky (4) V. K = [H] x [OH)
Answers
The equation that is NOT needed to solve for pH is option V: K = [H] x [OH].
Above equation represents the equilibrium constant for the autoionization of water (Kw) and is not directly relevant for calculating the pH of a weak-acid solution. To solve for the pH of a weak-acid solution using systematic treatment, we typically require equations related to the dissociation of the weak acid and the equilibrium expressions for the acid and its conjugate base. These equations allow us to set up a system of equations equal to the number of unknown concentrations, which can then be solved to determine the pH. Equations I, II, III, and IV are all relevant in the systematic treatment for calculating the pH of a weak-acid solution. They involve the concentration of the weak acid ([HA]), its dissociation into its conjugate base ([A-]) and hydrogen ions ([H]), and the equilibrium constant (Ka) associated with the weak acid dissociation.
To learn more about weak-acid click here: brainly.com/question/29833185
#SPJ11
in a lead chloride (pbcl2) saturated solution at an elevated temperature, the chloride ion concentration, [cl-], is 4.20 * 10^-2 m. what is the value of ksp for pbcl2 at this temperature?
Answers
To learn about the saturated solution and concentration to find the value of Ksp.
What is saturated solution?
When a solution has dissolved all of the solute it is capable of removing, it is said to be saturated. At a certain temperature, a saturated solution cannot dissolve any more solute. We can create a saturated solution by continuing to dissolve the solute until no more can be done.
What is concentration?
In chemistry and related fields, the idea of concentration is used frequently. It is a way to gauge how much of one substance is incorporated into another.
The ionic compound lead(II) chloride, or PbCl2, does not completely dissociate into lead(II) cations and chloride anions when placed in aqueous solution.
Instead of completely dissociating, a state of equilibrium will be established between the solid lead (II) chloride and the dissolved ions under the control of the solubility product constant, K sp.
PbCl 2(s) + 2 Cl (aq] Pb 2 + (aq]
The compound's molar solubility, or s in moles, represents the amount of lead(II) chloride that will dissolve in aqueous solution at a given temperature.
A mole of lead II chloride will result in the production of 1 mole of lead II cations and 2 moles of chloride anions. To determine the molar solubility of the solid, use an ICE table.
PbCl 2(s) + 2 Cl (aq] Pb 2 + (aq]
I 0 0
C (+s) (+2s)
E s 2s
According to the definition, the solubility product constant will equal
Ksp = (pb²⁺) (cl⁻)²
Ksp = s⋅ (2s)²= 4s³
As a result, lead (II) chloride will have a molar solubility of
4s³=1.6⋅10⁻⁵⇒ s= \(\sqrt[3]{1.6/4*10^-5}\) = 0.0159 M.
Therefore, the value of ksp for pbcl2 at this temperature is 0.0159 M.
Learn more about saturated solution from the given link.
https://brainly.com/question/1851822
#SPJ4
The mechanism of glyceraldehyde-3-phosphate dehydrogenase does NOT involveA phosphorylation of the substrate using ATP.B oxidation and phosphorylation of the substrate.C a covalent intermediate.D an active site histidine to serve as a proton acceptor.
Answers
The mechanism of glyceraldehyde-3-phosphate dehydrogenase does NOT involve a covalent intermediate. Hence, the correct option is (C).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is an enzyme involved in the glycolytic pathway, which converts glucose into pyruvate. The enzyme catalyzes the conversion of glyceraldehyde-3-phosphate (G3P) into 1,3-bisphosphoglycerate (1,3-BPG), coupled with the reduction of NAD+ to NADH. The mechanism of GAPDH involves several steps, but it does not involve phosphorylation of the substrate using ATP. Instead, the enzyme uses a covalent intermediate to transfer a hydride ion from G3P to NAD+, forming NADH. The covalent intermediate is formed between the Cys149 residue of the enzyme and the carbonyl group of G3P. This intermediate is then oxidized by the NAD+, resulting in the formation of 1,3-BPG and the release of NADH. In addition to the covalent intermediate, the mechanism of GAPDH involves the use of an active site histidine residue (His178) to serve as a proton acceptor. This histidine residue accepts a proton from the Cys149 residue during the formation of the covalent intermediate, and then donates a proton to the carbonyl group of G3P during the subsequent oxidation reaction.
To know more about covalent please refer: https://brainly.com/question/10777799
#SPJ4
The chemical equation below shows the formation of aluminum oxide (Al2O3) from aluminum (Al) and oxygen (O2).
4Al + 3O2 2Al2O3
The molar mass of O2 is 32.0 g/mol. What mass, in grams, of O2 must react to form 3.80 mol of Al2O3?
60.8
81.1
122
182
Answers
Answer:
Mass = 182.4 g
Explanation:
Given data:
Number of moles of Al₂O₃ = 3.80 mol
Mass of oxygen required = ?
Solution:
Chemical equation:
4Al + 3O₂ → 2Al₂O₃
Now we will compare the moles of aluminum oxide and oxygen.
Al₂O₃ : O₂
2 : 3
3.80 : 3/2×3.80 = 5.7
Mass of oxygen:
Mass = number of moles × molar mass
Mass = 5.7 mol × 32 g/mol
Mass = 182.4 g
Por qué el atomismo de balton se le considera un teoría científica mientras que a leucipio y Demócrito no?
Answers
Answer:
Because Democritus or Liucipius cannot demonstrate or proof their ideas as they did not have any equipment or any research to prove the existence of atoms.
Explanation:
John Dalton, Democritus and Leucipius are some of the greatest scientist and scholars of the past.
Democritus originally proposed or gave the idea of the of the composition of the matter of indivisible and tiny particles. John Dalton is credited for the beginning of the modern atomic theory.
Democritus believed that a matter is made up of atoms that can move about empty spaces. They are small, indestructible, solid, indivisible and of different shapes and sizes. Democritus proposed his idea at that time as there were no scientific advancement or instruments to prove his ideas about atoms.
Later on when science and scientific processes were advanced, Dalton was able to prove and proceed on the atomic model theory.
Democritus cannot prove his ideas as there were no instruments or advance scientific processes and so people felt his ideas as illogical. His proposals were based on his ideas.
Jane and Jack have a bicycling competition Jane rides a constant speed of 12 miles per hour while Jack rides at a constant speed of 330 feet per minute. How fast are Jane and Jack going in meters per second? Who finishes first? Show your factor-label method work. There are 5280 feet in one mile. There are 3.281 feet in one meter
Answers
Answer:
Explanation:
I don't know how you want the conversion done. I use dimensional analysis.
Jane
12 miles / hour [1.6 km/1 mile][1000 m/1 km][1 hour / 3600 sec]
12 * [1.6 * 3600 / 1000 m/s] = 69.12 m/s
5.33 m/s This answer is a bit shorter than using 5280 feet.
Using 5280 feet
12 miles / hour [5280 ft/1 mile] [1 m/3.281 feet] * [1 hr/3600 sec]
12 * 5280 / (3.281 * 3600)
12 *. 4470
5.36
Jack
330 feet / minute [ 1 meter / 3.281 feet] [1 minute / 60 seconds]
330 * 1/(3.281 * 60)
330 * 1/(196.86)
1.676 m/s
She's going faster than he is, no matter which method is used to do the calculation
why use naoh in determination of the acetylsalicylic acid uv spectroscopy
Answers
The use of sodium hydroxide (NaOH) in the determination of acetylsalicylic acid (ASA) by UV spectroscopy is for the purpose of removing the acetyl group from ASA, converting it to salicylic acid (SA).
This step is necessary because salicylic acid is more easily analyzed by UV spectroscopy, as it has a strong absorption peak at around 270 nm. In contrast, ASA has a weak absorption at this wavelength and is not easily detected by UV spectroscopy.
By adding NaOH to the solution of ASA, the acetyl group is deprotonated and can be removed by reaction with the hydroxide ion, converting the ASA to SA.
The resulting SA solution can then be measured by UV spectroscopy, providing a much stronger signal and more accurate determination of the ASA concentration.
In this manner, the use of NaOH allows for a more sensitive and accurate determination of the ASA concentration by UV spectroscopy.
Know more about uv spectroscopy
https://brainly.com/question/6476739#
#SPJ11
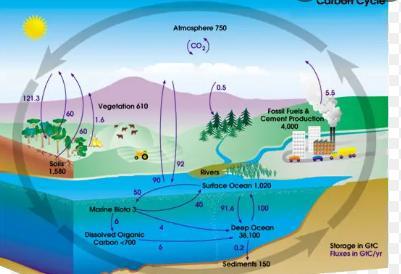
The combination of fossil fuel use and deforestation has emitted approximately 480 gigatons of carbon over the last century, but the amount of carbon in the atmosphere has only increased by approximately 190 gigatons. About 110 gigatons of this missing carbon went into which reservoir of the carbon cycle?.
Answers
About 110 gigatons of this missing carbon went into water reservoir of the carbon cycle.
What is carbon cycle?The biogeochemical cycle in which carbon is exchanged between the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth is known as the carbon cycle.
According to the question, emitted amount of carbon is 480 gigatons but the amount of carbon in the environment is approx 190 gigatons, so the remaining amount of the carbon was stoored by the oceans and other water reservoir, which may be get clear throug the attached diagram.
Hence remaining gigatons of carbon went into water reservoir.
To know more about carbon cycle, visit the below link:
https://brainly.com/question/24293689
#SPJ1
you need to make an aqueous solution of 0.180 m potassium sulfide for an experiment in lab, using a 300 ml volumetric flask. how much solid potassium sulfide should you add?
Answers
4.2228 g of solid potassium sulfide should be added to make an aqueous solution of 0.180 M potassium sulfide for an experiment in lab, using a 300 ml volumetric flask.
The given molarity of the aqueous solution of potassium sulfide is 0.180 M and the volume of the solution is 300 mL. We are required to find out the amount of solid potassium sulfide required to make the solution.
The formula to calculate the number of moles is: Number of moles = Molarity x Volume (in liters) 1. Convert the volume into liters.300 mL = 0.3 L2. Substitute the given values in the above formula.Number of moles = 0.180 M x 0.3 LNumber of moles = 0.054 mol3. The molecular formula of potassium sulfide is K2S. It means there are two moles of K for one mole of K2S. Hence, we can calculate the moles of K.Number of moles of K = 2 x 0.054
Number of moles of K = 0.108 mol4. The molar mass of K is 39.1 g/mol. Hence, we can calculate the mass of K required to make 0.108 mol.Number of grams of K = Number of moles x Molar massNumber of grams of K = 0.108 mol x 39.1 g/mol
Number of grams of K = 4.2228 g. Hence, 4.2228 g of solid potassium sulfide should be added to make an aqueous solution of 0.180 M potassium sulfide for an experiment in lab, using a 300 ml volumetric flask.
To learn more about aqueous visit;
https://brainly.com/question/30215562
#SPJ11
What are chemical changes of matter?
Answers
Answer:
Chemical Changes are also called Chemical Reactions. Chemical reactions involve combining different substances. The chemical reaction produces a new substance with new and different physical and chemical properties. Matter is never destroyed or created in chemical reactions.
Explanation:
Looking at this rate law, which of the steps would be the rate determining step and why?

Answers
The step in a chemical reaction that defines the pace (or rate) at which the entire reaction occurs is known as the rate-determining step.
Thus, The rate-determining step is comparable to the funnel's neck. The breadth of the funnel's neck, not the pace at which water is poured into it, limits or determines how quickly water flows down a funnel.
The sluggish step of a reaction controls the rate of a reaction, much like the funnel's neck.
Not all reactions have rate-determining stages, and those that do only have them if one of their steps is noticeably slower than the others.
Thus, The step in a chemical reaction that defines the pace (or rate) at which the entire reaction occurs is known as the rate-determining step.
Learn more about Rate determining steps, refer to the link:
https://brainly.com/question/31809160
#SPJ1
What is the molality of a solution that contains 31.0 g HCI in 5.00 kg water?
Answers
Molar mass (H) + Molar mass (Cl) = 1.007 g/mol + 35.453 g/mol = 36.460 g/mol
Now, calculate the number of moles of HCl:
moles = mass / molar mass
moles = 31.0 g / 36.460 g/mol ≈ 0.850 mol
Next, calculate the mass of water (solvent) in kilograms:
mass of water = 5.00 kg
molality = moles of solute / mass of solvent (in kg)
molality = 0.850 mol / 5.00 kg ≈ 0.170 m
Explain the effect that increasing the nacl concentration have on osmotic pressure.
Answers
Increasing the NaCl concentration will increase the osmotic pressure.
Osmotic pressure, alongside the vapor pressure depression, freezing point depression and the boiling point elevation are the colligative properties od solution.
The direction of osmotic pressure is always from the side with the lower concentration of solute to the side with the higher concentration.
π = c(NaCl) × T(NaCL) × R
π is osmotic pressure
c is concentration of solution.
T is temperature in Kelvins.
R is universal gas constant.
Greater the concentration of sodium chloride, the greater is the osmotic pressure, because the osmotic pressure and the concentratio are in direct proportion.
More about osmotic pressure: brainly.com/question/5925156
#SPJ4
what would u add if the soil is too basic~~ ?
Answers
Slaked lime is the ans
thank me later : p
