Answers
Answer:the tendency of liquids to form vapors
Explanation
Answer:
the tendency of liquid to form vapors
Explanation:
yes
Related Questions
what are minerals?
explained
Answers
A mineral is a substance such as tin, salt, or sulphur that is formed naturally in rocks and in the earth. Minerals are additionally observed in small portions in food and drink.
Why are minerals important?Minerals are integral for three essential reasons: building robust bones and teeth. controlling physique fluids inside and outdoor cells. turning the meals you devour into energy.
Why are minerals so important?Minerals are necessary for your body to remain healthy. Your physique uses minerals for many one of a kind jobs, which include maintaining your bones, muscles, heart, and talent working properly. Minerals are additionally necessary for making enzymes and hormones. There are two types of minerals: macrominerals and hint minerals.
Learn more about minerals here;
https://brainly.com/question/15844293
#SPJ1
What causes blood cells to shrink?
Answers
What is the pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C? A) 4.48 x 10¹¹ atm B) 2.24 x 10⁰ atm C) 1.12 x 10³ atm D) 2.24 x 10³ atm
Answers
The pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C is 2.24 × 10⁰ atm.
How to calculate pressure?The pressure of a substance can be calculated using the following formula;
PV = nRT
P = pressureV = volumen = no of molesR = gas law constantT = temperatureAccording to this question, the pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C can be calculated as follows:
P × 2 = 0.1 × 0.0821 × 546
2P = 4.48266
P = 2.24 × 10⁰ atm
Learn more about pressure at: https://brainly.com/question/31525061
#SPJ1
Ice charts (Image)
Need help figuring how this works.

Answers
The equilibrium concentrations of the gases are:
[H2] = 0.865 M, [CO2] = 0.865 M, [CO] = 1.135M ,[H2O] = 1.135 M.
What is the sum of molar concentration of reactants?The sum of the molar concentrations of the reactants (H2 and CO2) is equal to the sum of the molar concentrations of the products (CO and H2O), as expected for a reaction at equilibrium.
The equilibrium concentrations of the four gases can be determined using the equilibrium constant expression and the initial concentrations of the reactants.
The equilibrium constant expression for the reaction is:
K_eq = [CO][H2O]/[H2][CO2]
where the concentrations are in units of M (molarity).
We are given that K_eq = 0.771 at 650 degrees Celsius.
We start by setting up an ICE table (Initial, Change, Equilibrium) to determine the equilibrium concentrations:
H2 CO CO H2O
Initial 2.00 2.00 0 0
Change -x -x +x +x
Equil. 2.00-x 2.00-x x x
where "x" is the change in concentration from the initial state to the equilibrium state, and we assume that the reaction proceeds to equilibrium.
Substituting the equilibrium concentrations into the equilibrium constant expression and solving for "x", we get:
K_eq = [CO][H2O]/[H2][CO2] = x^2 / (2.00 - x)^2 = 0.771
Solving for "x" using the quadratic formula, we get:
x = 1.135 M
Therefore, the equilibrium concentrations of the gases are:
[H2] = 0.865 M
[CO2] = 0.865 M
[CO] = 1.135 M
[H2O] = 1.135 M
To know more about reaction, visit:
https://brainly.com/question/28984750
#SPJ1
Larry made this picture to represent a chemical reaction:
Which of the following statements best explains the type of chemical reaction represented by Larry's picture?
a
It represents a synthesis reaction because two reactants form one product.
b
It represents a synthesis reaction because the total mass of the product is less than the mass of the reactants.
c
It represents a decomposition reaction because one reactant breaks apart and forms two products.
d
It represents a decomposition reaction because the same atoms are present in the reactants and products.

Answers
To solve such this we must know the concept of synthesis reaction. Therefore, the correct option is option A that is It represents a synthesis reaction because two reactants form one product.
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
It represents a synthesis reaction because two reactants form one product. This statements best explains the type of chemical reaction represented by Larry's picture. synthesis reaction is the one where two reactant combine to form one product.
Therefore, the correct option is option A.
Learn more about the chemical reactions, here:
https://brainly.com/question/3461108
#SPJ1
a. Phenol (C6H5OH) is an aromatic compound and a colourless liquid widely used in household products
and medicine. When it reacts with chlorine liquid (chlorine is a diatomic molecule), in the presence of a
catalyst, one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine
atom, and liquid chlorophenol is formed. This replacement process is called chlorination
Write a balanced chemical equation for the chlorination reaction and explain how you balanced it. Note
that hydrogen chloride gas (HCI) is also a product of this chemical reaction and you should ignore the
presence of the catalyst in the equation.
Answers
Because there are 2 Cl on the left, we will put a coefficient 2in front of HCl on the right side to balance out the Cl. This would result in an unequal amount of H, with 6 on the right side and 7 in the left, so we have to put a coefficient of 2 in front of C6H5OH and C6H4OH on both sides to balance out the H. By doing this, we would obtain an equal amount of H on both sides. The Carbon is already balanced, and so is the Oxygen.
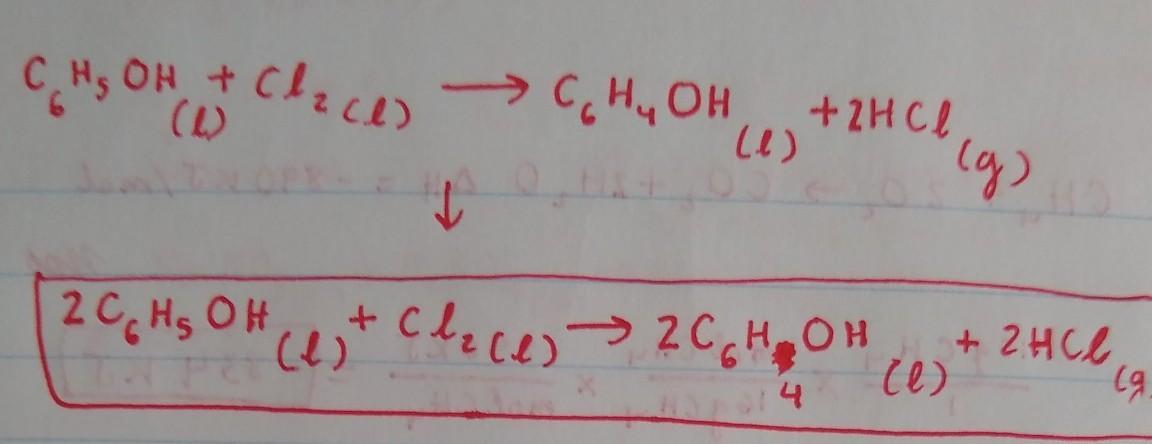
The balanced chemical reaction equation for the reaction between aromatic phenol and chlorine gas in the presence of FeCl3 as catalyst is as follows;
C6H6O + Cl2 -------> C6H5OCl + HCl
An aromatic compound has 4n + 2 number of pi electrons. This condition is satisfied by phenol. Hence, phenol has the stability associated with aromatic compounds.
The reaction of phenol with chlorine in the presence of a catalyst such as FeCl3 is an aromatic electrophillic substitution reaction.
This reaction yields a chlorinated phenol (one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine atom and chlorophenol is formed).
A balanced chemical reaction equation is one in which the number of atoms of each element at the reactant side and the product side are equal. This condition is satisfied for the reaction;
C6H6O + Cl2 -------> C6H5OCl + HCl
Learn more; https://brainly.com/question/6170291
Which state of matter has a definite volume and takes the shape of its container?
liquid
gas
solid
Answers
Answer:
solid
Explanation:
liquid can't take the shape of the container because it flows freely and it doesn't have a definite shape and it has space between it e.g water
gas also doesn't shape and e. g air
but solid takes a definite shape because it doesn't have space in between of
Answer:
sold
Explanation:
The figure below shows a walkway with a handrail. Angle is the angle between the walkway and the horizontal, while angle is the angle between the vertical posts of the handrail and the walkway. Use the figure below to work the problem. (Assume that the vertical posts are perpendicular to the horizontal.)
Are angles and complementary or supplementary angles?
complementary
supplementary
Answers
The angles as shown are supplementary angles because the add up to 180 degrees.
What are supplementary angles?Two angles are said to be supplementary if they add up to 180 degrees. Now we know that the sum of angles on straight line is 180 degrees. If we look at the image as shown in the image attached, we can see that the angles lie on a straight line.
As such, we can conclude that the angles as shown are supplementary angles because the add up to 180 degrees.
Learn more about supplementary angles:https://brainly.com/question/13045673
#SPJ1

If you placed 413g of Bal2 in a beaker and filled it with water to a total volume of 750ml, calculate the molarity of the solution
Answers
To calculate the molarity of a solution, we need to determine the number of moles of the solute (Bal2) and then divide it by the volume of the solution in liters.
Given:
Mass of Bal2 = 413 g
Volume of solution = 750 ml = 0.75 L
1. Calculate the number of moles of Bal2:
First, we need to convert the mass of Bal2 to moles using its molar mass. The molar mass of Bal2 can be calculated by summing the atomic masses of boron (B) and iodine (I):
Molar mass of Bal2 = (atomic mass of B × 1) + (atomic mass of I × 2)
Molar mass of Bal2 = (10.81 g/mol × 1) + (126.90 g/mol × 2)
Molar mass of Bal2 = 10.81 g/mol + 253.80 g/mol
Molar mass of Bal2 = 264.61 g/mol
Now we can calculate the number of moles of Bal2:
Moles of Bal2 = Mass of Bal2 / Molar mass of Bal2
Moles of Bal2 = 413 g / 264.61 g/mol
Moles of Bal2 ≈ 1.561 mol
2. Calculate the molarity of the solution:
Molarity (M) = Moles of solute / Volume of solution (in liters)
Molarity (M) = 1.561 mol / 0.75 L
Molarity (M) ≈ 2.081 M
Therefore, the molarity of the solution is approximately 2.081 M.
The molarity of the solution is approximately 1.408 M as to calculate the molarity of a solution, one must need to know the number of moles of the solute and the volume of the solution in liters.
The molar mass of BaI₂ is:
Ba (barium) atomic mass = 137.33 g/mol
I (iodine) atomic mass = 126.90 g/mol
Molar mass of BaI₂ = (Ba atomic mass) + 2 × (I atomic mass)
= 137.33 + 2 × 126.90
= 137.33 + 253.80
= 391.13 g/mol
Given that the mass of BaI₂ is 413 g,
Number of moles = Mass / Molar mass
= 413 g / 391.13 g/mol
= 1.056 moles
Volume of solution = 750 ml = 750/1000 = 0.75 L
Finally, one can calculate the molarity of the solution using the formula:
Molarity = Number of moles / Volume of solution
= 1.056 moles / 0.75 L
= 1.408 M
Learn more about molarity here.
https://brainly.com/question/13386686
#SPJ1
What a balanced chemical equation for the single displacement reaction you observed in Experiment 3. Include physical states.
Answers
The __________ stage of Kohlberg’s postconventional level focuses on making judgments based on what best protects individuals.
A.
marketplace morality
B.
social contract
C.
law and order
D.
good boy/good girl
Answers
Answer:
the answer is B- social contract
Explanation:
the person above me is correct
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
please help i’ll give brainliest

Answers
28 mL
it counts by 2 from 20
Answer:
28 ml
Explanation:
28 ml
my aunt told me
⚠️Which type of energy warms earths surface⚠️ ASAP needed 10points

Answers
Solar energy is the type of energy that warms earth surface. Details about solar energy can be found below.
What is solar energy?Solar energy is the energy in the form of electromagnetic radiation emitted from the Sun.
Solar energy is especially that part of electromagnetic energy that is converted into usable thermal or electrical energy by man.
This energy from the sun warms the Earth surface and provides the heat needed for various activities.
Learn more about solar energy at: https://brainly.com/question/9704099
#SPJ1
CuBr2 percent composition
Answers
The percent composition of CuBr₂ is approximately 28.46% of Cu and 71.54% of Br.
To determine the percent composition of CuBr₂ (copper(II) bromide), we need to calculate the mass of each element in the compound and then divide it by the molar mass of the entire compound.
The molar mass of CuBr₂ can be calculated by adding up the atomic masses of copper (Cu) and bromine (Br) in the compound. The atomic masses of Cu and Br are approximately 63.55 g/mol and 79.90 g/mol, respectively.
Molar mass of CuBr₂ = (63.55 g/mol) + 2(79.90 g/mol) = 223.35 g/mol
Now, let's calculate the percent composition of each element in CuBr₂:
Percent composition of copper (Cu):
Mass of Cu = (63.55 g/mol) / 223.35 g/mol × 100% ≈ 28.46%
Percent composition of bromine (Br):
Mass of Br = 2(79.90 g/mol) / 223.35 g/mol × 100% ≈ 71.54%
Therefore, the percent composition of CuBr₂ is approximately:
- Copper (Cu): 28.46%
- Bromine (Br): 71.54%
These values represent the relative mass percentages of copper and bromine in the compound CuBr₂.
for more such questions on composition
https://brainly.com/question/28250237
#SPJ8
What is the molarity of a solution containing 5.035 grams of FeCIe in enough water to make 500 mL of solution?
Answers
The molar mass of FeCl2 is 126.75 g/mol (55.85 g/mol for Fe and 35.45 g/mol for Cl).
Number of moles of FeCl2 = mass of FeCl2 / molar mass of FeCl2
= 5.035 g / 126.75 g/mol
= 0.0397 mol
Now we can calculate the molarity of the solution:
Molarity = moles of solute / liters of solution
Since we have 500 mL of solution, we need to convert it to liters by dividing by 1000:
Liters of solution = 500 mL / 1000 mL/L
= 0.5 L
Now we can calculate the molarity:
Molarity = moles of solute / liters of solution
= 0.0397 mol / 0.5 L
= 0.0794 M
Therefore, the molarity of the solution containing 5.035 grams of FeCl2 in enough water to make 500 mL of solution is 0.0794 M.
coenzyme q carries electrons between which stages of the electron-transport chain? check all that apply.
Answers
Coenzyme q carries electrons from complex I to complex III and complex II to complex III in the electron-transport chain.
Coenzyme q (CoQ), also known as ubiquinone, is the electron carrier in the electron transport system (ETS) present on the inner membrane of mitochondria.
Ubiquinone is a ubiquitous quinone, which accepts electrons from complex II ( succinate dehydrogenase) and reduces to ubiquinol ( reduced form)
The purpose of the ETS is to generate an H+ ion concentration, by carrying electrons obtained from NADH AND \(FADH_{2}\) produced by the Krebs cycle and glycolysis in the mitochondrial matrix. This H+ ion potential will be used by ATP synthase to generate ATP.
To know more about ETS :
https://brainly.com/question/876880
https://brainly.com/question/18686654
The inner mitochondrial membrane contains CoQ, a key part of the mitochondrial electron transport chain (ETC), which moves electrons from complexes I and II to complex III to provide energy for proton translocation to the intermembrane gap.
What is mitochondria ?An organelle called a mitochondrion can be found in the cells of the majority of Eukaryotes, including mammals, plants, and fungi. Adenosine triphosphate, which is produced by aerobic respiration in mitochondria with their double membrane structure, is used as a source of chemical energy throughout the entire cell.
Coenzyme Q10 takes electrons from reducing equivalents produced during the metabolism of fatty acids and glucose and then transports them to electron acceptors as part of the mitochondrial electron transport chain.
Ubiquinone, also known as coenzyme Q, is a lipophilic molecule that is found in all tissues and cells and is mostly found in the inner mitochondrial membrane. It is generally known that Coenzyme Q is an essential part of the oxidative phosphorylation process in mitochondria.
Thus, The inner mitochondrial membrane contains CoQ, a key part of the mitochondrial electron transport chain (ETC).
To learn more about mitochondria, follow the link;
https://brainly.com/question/10688306
#SPJ12
Label the element from which it is easier to remove an electron argon or bromine
Answers
The element from which it is easier to remove an electron between Argon and Bromine is Bromine.
This can be explained by electron affinity.
Electron affinity is defined as amount of the energy released when one mole of electron is added to one mole of atom or to the outermost or valence shell to form anion. It is the measure of attraction existing between atom and electron.
Electron affinity of Bromine is 324.537 kJ/mol and electron affinity of Argon is -96 kJ/mol. A higher electron affinity indicates that an atom accepts or removes the electrons more easily and low electron affinity means that an atom do not accept or remove electrons as easily.
Bromine has atomic number 35, which means it has 35 protons in nucleus. It has 35 electrons and for a bromine atom to become a 1- bromide ion, it will have to gain an electron. If it loses electrons, it receives a positive charge because it has more protons than electrons.
Argon is a noble gas element. It means argon is highly stable because it has full outer shell of electrons. Argon has low affinity. Therefre, it does not lose or gain electrons because it is already stable.
To learn more about electron affinity,
brainly.com/question/11562585
#SPJ1
Lead ions are toxic when absorbed into the body and can interfere with the neurological development of children. Based on what you learned in this lab activity, what substance might be added to an IV solution to remove Pb2 ions from the blood of a contaminated person
Answers
Answer:
The interpretation of the particular subject is covered in the subsection below in detail.
Explanation:
Large quantities of heavy substances like Lead ions become extremely poisonous when provided by a human. The administration of the medications recognized as "chelators" will eliminate these harmful chemicals from an infected individual's blood.However, here law enforcers calcium sodium polyacrylate seems to be the safest chelator in radiation sickness. It could be administered intravenously and attaches throughout the blood system with either the lead ions and afterward, removes the metal-chelator complicated from urine.what is a formula car? (This is for my chemistry project)
Answers
Answer:
A formula car is a single seat, open cockpit, open wheel racing car with substantial front and rear wings and an engine positioned behind the driver intended to be used in competition.
Gizmo Warm-up Just like students sharing markers, atoms sometimes share or swap electrons. By doing this, atoms form bonds. The Ionic Bonds Gizmo allows you to explore how ionic bonds form. To begin, check that Sodium (Na) and Chlorine (Cl) are selected from the menus at right. Click Play ( ) to see electrons orbiting the nucleus of each atom. (Note: These atom models are simplified and not meant to be realistic.) 1. Each atom consists of a central nucleus and several shells that contain electrons. The outermost electrons are called valence electrons. How many valence electrons does each atom have
Answers
Answer: Sodium element has 1 valence electron and chlorine element has 7 valence electrons.
Explanation:
Valence electrons are defined as the electrons that are present in the outermost shell.
An ionic compound is formed when the complete transfer of electrons takes place from one element (usually metals) to another element (usually non-metals).
To know this, we need to write the electronic configuration of each element.
Sodium is the 11th element of the periodic table and has an electronic configuration of \(1s^22s^22p^63s^1\)
It has 1 valence electron
Chlorine is the 17th element of the periodic table and has an electronic configuration of \(1s^22s^22p^63s^23p^5\)
It has 7 valence electrons
Hence, sodium element has 1 valence electron and chlorine element has 7 valence electrons.
Which word names melted rock and minerals found beneath Earth's crust?
A. lava B. rhyolite C. magma D. gabbro
Answers
Answer:
magma
Explanation:
magma is the stuff under the earth's surface, from the greek word mágma, which means thick unguent.
magma has been found on other planets!
evidence:

Hazmat Poison gas is in what class?
Answers
HAZMAT Class 6 Toxic and infectious substances.
Answer:
CLASS 2 Gases
Explanation:
Hope this helps
calculate the pH of the solution obtained if 40cm^3 of 0.2M HCl was added to 30cm^3 of 0.1M NaOH
Answers
To calculate the pH of the solution obtained by mixing HCl and NaOH, we need to consider the neutralization reaction between the two compounds. The reaction between HCl (hydrochloric acid) and NaOH (sodium hydroxide) produces water (H₂O) and forms a salt (NaCl).
Given:
Volume of HCl solution (V₁) = 40 cm³
Concentration of HCl solution (C₁) = 0.2 M
Volume of NaOH solution (V₂) = 30 cm³
Concentration of NaOH solution (C₂) = 0.1 M
1. Determine the moles of HCl and NaOH used:
Moles of HCl = Concentration (C₁) × Volume (V₁)
Moles of HCl = 0.2 M × 0.04 L (converting cm³ to L)
Moles of HCl = 0.008 mol
Moles of NaOH = Concentration (C₂) × Volume (V₂)
Moles of NaOH = 0.1 M × 0.03 L (converting cm³ to L)
Moles of NaOH = 0.003 mol
2. Determine the limiting reagent:
The stoichiometry of the reaction between HCl and NaOH is 1:1, meaning that they react in a 1:1 ratio. Whichever reactant is present in a smaller amount will be the limiting reagent.
In this case, NaOH is present in a smaller amount (0.003 mol), which means it will be fully consumed during the reaction.
3. Determine the excess reagent and its remaining moles:
Since NaOH is the limiting reagent, we need to find the remaining moles of HCl.
Moles of HCl remaining = Moles of HCl initially - Moles of NaOH reacted
Moles of HCl remaining = 0.008 mol - 0.003 mol
Moles of HCl remaining = 0.005 mol
4. Calculate the concentration of HCl in the resulting solution:
Volume of resulting solution = Volume of HCl solution + Volume of NaOH solution
Volume of resulting solution = 0.04 L + 0.03 L
Volume of resulting solution = 0.07 L
Concentration of HCl in the resulting solution = Moles of HCl remaining / Volume of resulting solution
Concentration of HCl in the resulting solution = 0.005 mol / 0.07 L
Concentration of HCl in the resulting solution ≈ 0.071 M
5. Calculate the pH of the resulting solution:
pH = -log[H⁺]
pH = -log(0.071)
Using logarithm properties, we can determine the pH value:
pH ≈ -log(0.071)
pH ≈ -(-1.147)
pH ≈ 1.147
Therefore, the pH of the solution obtained by mixing 40 cm³ of 0.2 M HCl and 30 cm³ of 0.1 M NaOH is approximately 1.147.
Explain how this chemical equation demonstrates the Law of Conservation of Matter (Mass).
Answers
Answer:
Explanation:
The equation must be balanced on both sides to show the law of conservation of mass. So, as long as it is equaled, the law is shown.
Which best describes the energy change that takes place during deposition?
Heat energy released by the substance
Heat energy is maintained by the substance
Heat energy is slowly gained by the substance
Heat energy is quickly absorbed by the substance
Answers
Answer:
heat energy released by the substance
The energy change that takes place during deposition is that heat energy is released by the substance. This statement is true about deposition.
Deposition is a phase transition that occurs when a gas is converted directly to a solid without passing through the liquid state. During deposition, energy is released by the gas particles and absorbed by the surface, resulting in a decrease in the energy of the gas particles and an increase in the energy of the surface particles.As a result, the substance releases heat energy as it changes from a gas state to a solid state.
Therefore, the correct option is:Heat energy released by the substance.
Learn more about heat energy,here:
https://brainly.com/question/29210982
#SPJ5
A certain liquid has a normal boiling point of and a boiling point elevation constant . A solution is prepared by dissolving some glycine () in of . This solution boils at . Calculate the mass of that was dissolved. Round your answer to significant digit.
Answers
The question is incomplete, the complete question is:
A certain substance X has a normal freezing point of \(-6.4^oC\) and a molal freezing point depression constant \(K_f=3.96^oC.kg/mol\). A solution is prepared by dissolving some glycine in 950. g of X. This solution freezes at \(-13.6^oC\) . Calculate the mass of urea that was dissolved. Round your answer to 2 significant digits.
Answer: The mass of glycine that can be dissolved is \(1.3\times 10^2g\)
Explanation:
Depression in the freezing point is defined as the difference between the freezing point of the pure solvent and the freezing point of the solution.
The expression for the calculation of depression in freezing point is:
\(\text{Freezing point of pure solvent}-\text{freezing point of solution}=i\times K_f\times m\)
OR
\(\text{Freezing point of pure solvent}=\text{Freezing point of solution}=i\times K_f\times \frac{m_{solute}\times 1000}{M_{solute}\times w_{solvent}\text{(in g)}}\) ......(1)
where,
Freezing point of pure solvent = \(-6.4^oC\)
Freezing point of solution = \(-13.6^oC\)
i = Vant Hoff factor = 1 (for non-electrolytes)
\(K_f\) = freezing point depression constant = \(3.96^oC/m\)
\(m_{solute}\) = Given mass of solute (glycine) = ?
\(M_{solute}\) = Molar mass of solute (glycine) = 75.07 g/mol
\(w_{solvent}\) = Mass of solvent = 950. g
Putting values in equation 1, we get:
\(-6.4-(-13.6)=1\times 3.96\times \frac{m_{solute}\times 1000}{75.07\times 950}\\\\m_{solute}=\frac{7.2\times 75.07\times 950}{1\times 3.96\times 1000}\\\\m_{solute}=129.66g=1.3\times 10^2g\)
Hence, the mass of glycine that can be dissolved is \(1.3\times 10^2g\)
Which defines the average inetic energy of a system's particles?
O density
O pressure
O temperature
O volume
Answers
Temperature
Explanation:
Because Temperature is a measure of the average kinetic energy of all the molecules in a gas. As the temperature and, therefore, kinetic energy, of a gas changes, the RMS speed of the gas molecules also changes. The RMS speed of the molecules is the square root of the average of each individual velocity squared.
Read the given equation.
2Na+ 2H₂O 2NaOH + H₂
During a laboratory experiment, a certain quantity of sodium metal reacted with water to produce sodium hydroxide and hydrogen gas. What was the initial quantity of
sodium metal used if 7.80 liters of H₂ gas were produced at STP?
07:29 grams
09.30 grams
12.2 grams
16.0 grams
Answers
How is the temperature scale different than a typical thermometer?