what does the formnation of yellow color in the tube mean? what events lead to
Answers
The formation of yellow color in the tube indicates that the reaction has occurred between the enzyme and substrate.
This color change is the that helps to indicate whether the reaction has taken place or not.The enzymes can act as catalysts that enhance the rate of chemical reactions by decreasing the activation energy required for a particular reaction to occur.The formation of yellow color in the tube usually indicates that a particular chemical reaction has occurred between an enzyme and substrate. For instance, if a reaction occurs, the yellow color can be caused by the production of the product. Conversely, if no reaction occurs, then the absence of the yellow color indicates no reaction occurred.
In enzyme assays, we usually measure the rate of the reaction by monitoring the production of the product, which is usually indicated by a color change. This color change is the that helps to indicate whether the reaction has taken place or not. Therefore, the formation of yellow color is usually an important aspect in most enzyme assays.
To know more about enzyme visit:
https://brainly.com/question/30361820
#SPJ11
Related Questions
The elements from this section of the periodic table all belong to the same
A. Family
B. Group
C. Period
D. Valence
Plz help!!

Answers
Answer:
C. Period
Explanation:
The elements from this section of the periodic table belongs to the same period.
A period on the periodic table is a horizontal arrangement of elements according to their atomic number.
A group or family is the vertical organization of elements. Valence is the number of outer shell electrons an atom possess.What kind of reaction is this
Na2O + CO2 → Na2CO3
Answers
Answer:
Explanation:
Na 2O + CO 2 → Na 2CO. 3
This is an acid-base reaction (neutralization): Na 2O is a base, CO 2 is an acid.
Hope this helps!!!!!!
Brainlist please
The reaction of N₂O with CO₂ giving Na₂CO₃ is a combination reaction, where two reactants combines to form a product.
What are types of reactions?There are different kinds of reactions namely, displacement reactions, combination reactions, decomposition reaction etc.
The reaction in which one reactant species displace the constituent group of another reactant it is termed as displacement reactions. On decomposition reaction, one reactant decomposes into two or more products.
In a single displacement reaction, one of the group is displaced to form a new product and in double displacement reactions, two species from reactant side are displaced.
Similarly, a reaction in which two reactants simply combines to form a product is termed as combination reaction.
Therefore, the type of the given reaction is combination.
To learn more about types of reactions, refer the link below:
https://brainly.com/question/24762458
#SPJ2
give the nuclear symbol, including superscript and subscript, for each type of radiation.
alpha particle: He beta particle: gamma ray: Y Which type of radiation has a negative charge? a. alpha b. beta
c. gamma Which type of natural radiation is pure energy? a. gamma b. alpha
c. beta
Which type of natural radiation has the most massive particles? a. beta b. alpha c. gamma
Answers
The subscript in alpha radiation stands for the charge on the nucleus (i.e., the quantity of protons or atomic number, also known as the Z number), and the superscript is the mass number.
In beta radiation, the particle's charge is indicated by the subscript -1, and its almost completely absent mass is indicated by the superscript 0. (no protons or neutrons). The radioactive thorium-234 nucleus is another illustration.
Alpha, beta, and gamma are what?While alpha and beta particles have positive and negative charges, respectively, gamma rays are neutral. An alpha particle is created when two protons and two neutrons come together. Beta particles are high-energy electrons. Photons, which are electromagnetic energy waves, are gamma rays.
To know more about alpha radiation visit:-
https://brainly.com/question/6070167
#SPJ4
A box has a mass of 150kg. If a net force of 3000N acts on the box, what is the box's acceleration?
Answers
Answer:
3000 ÷ 150
Explanation:
acceleration = N/kg
Can somebody plz help answer this question correctly thank you! ONLY 1-2 sentences is good thanks!
WILL MARK BRAINLIEST WHOEVER ANSWERS THIS QUESTION :DDD

Answers
to generate energy means to create energy. chemical energy is the energy that is created when you put effort into doing something..
what separation procedure is most likely to happen? .a. freezing a compound will break it down into elements .b. filtering a mixture will separate it into mixtures .c. boiling a pure substance will separate it into mixtures .d. melting an element will break it down into compounds?
Answers
Explanation:
i think its B but i'm not fully sure (Correct)
Answer:
B.
Explanation:
A chemistry student combines 23.0 grams of sodium and 71.0 grams of chloride to form sodium chloride. According to the law of conservation of mass, what mass of sodium chloride should be formed if the reaction proceeds to completion?
Answers
Answer:
94.0 g.
Explanation:
Hello!
In this case, since the lay of conservation of mass states that energy cannot be neither created nor destroyed, during a chemical reaction it is seen that the reactions undergo a change by which bonds can be broken or formed depending on the case. Thus, for the formation of sodium chloride we evidence the formation of the Na-Cl bond which means sodium is combined with chlorine according to the following chemical reaction:
\(2Na(s)+Cl_2(g)\rightarrow 2NaCl(s)\)
It means that the reuslting mass of product is:
\(m_{NaCl}=23.0+71.0g=94.0g\)
Best regards!
6) How many grams of CCl4 are present in 2.67 moles of CCl4?
Answers
Answer:410.6 g
Explanation:

To what temperature must 20.2 g of hydrogen gas be heated at 110 kPa to occupy a volume of 4.45 x 10^-2 kL
Answers
Answer:
59K
Explanation:
Using the general gas law equation:
PV = nRT
Where;
P = pressure (atm)
V = volume (L)
n = number of moles (mol)
R = gas constant (0.0821 Latm/molK)
T = temperature (K)
According to the information given in the question;
- mass of hydrogen gas = 20.2g
mole = mass/molar mass
mole = 20.2/2.015
mole = 10.01mol
- Pressure = 110 Kpa
1 kilopascal = 0.00987 atmosphere
110kPa = 110 × 0.00987
= 1.09atm
- Volume = 4.45 x 10^-2 kL
1 kL = 1000L
4.45 x 10^-2 kL = 4.45 x 10^-2 × 10³
4.45 × 10¹
= 44.5L
Using PV = nRT
T = PV/nR
T = 1.09 × 44.5/0.0821 × 10.01
T = 48.505/0.821
T = 59.02
T = 59K
I need the definition of Friction, choose one! Thank you

Answers
Answer:
the force resisting motion. it slows things down
A blu-ray laser has a wavelength of 405nm. In what region of the electromagnetic spectrum is this radiation? What is its frequency?
Answers
Answer:
It lies in in the visible region.
Frequency: is 7.407 × 10^-14 Hz
\({ \bf{V = f \lambda}} \\ { \tt{3.0 \times {10}^{8} = f \times (405 \times {10}^{ - 9}) }} \\ { \tt{f = 7.407 \times {10}^{14} \: hertz}}\)
Explanation:
V is speed of light.
f is frequency
lambda is the wavelength
What is the approximate ph of a 0.10 m solution of a weak acid that has a ka of 5 x 10-5 m?
A) 2.7 PH = -Lay [At] B) 5.3 [ PH= Pkat Lug C) 4.8 О. Том D) 11.3 Pka = 4.30 Oka=-Lag Ka Pka= -Log
Answers
The approximate pH of a 0.10 M solution of a weak acid with a Ka of 5x10-5 M is 4.8.
To calculate the approximate pH of a weak acid solution, we can use the Henderson-Hasselbalch equation, which states that pH = pKa + log([A-]/[HA]).
In this equation, [A-] is the concentration of the conjugate base and [HA] is the concentration of the weak acid. In this case, [HA] is 0.10 M and [A-] is 5x10-5 M.
Calculate the molarity of the weak acid:
Molarity = 0.10 m (given)
Calculate the Ka of the weak acid:
Ka = 5 x 10-5 m (given)
Calculate the concentration of the conjugate base:
Conjugate base concentration = Ka x molarity = 5 x 10-5 m x 0.10 m = 5 x 10-6 m
Calculate the pH of the solution:
pH = -log[conjugate base] = -log(5 x 10-6) = 4.8
For more questions like pH click the link below:
https://brainly.com/question/15289741
#SPJ4
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
What is meant by N 2? A.OTwo nitrogen atoms formed a molecule. B.OTwo nitrogen atoms form a compound. C.The atomic number of nitrogen is two. D.The atomic mass of nitrogen is two.
Answers
The correct answer is A. "N₂" represents two nitrogen atoms that have formed a molecule.
N₂ refers to a diatomic molecule composed of two nitrogen atoms bonded together by a strong triple covalent bond. Nitrogen (N) is an element with an atomic number of 7, meaning it has seven protons in its nucleus.
Each nitrogen atom has five valence electrons in its outermost electron shell. To achieve a stable electron configuration, two nitrogen atoms can share three pairs of electrons, resulting in the formation of the N₂ molecule.
Option B is incorrect because compounds are formed when different elements combine chemically, whereas N₂ consists of only one element, nitrogen. Option C is incorrect because the atomic number of nitrogen is 7, not 2. Option D is incorrect because the atomic mass of nitrogen is approximately 14 atomic mass units (AMU), not 2.
Therefore, N₂ represents two nitrogen atoms that have bonded together to form a molecule, which is the most accurate description among the given options.
Learn more about atomic mass units here:
https://brainly.com/question/32369855
#SPJ11
I need help on this please

Answers
Answer:
D looks right to me, but if I'm wrong then sorry
The gravitational pull between two objects depends on their mass and distance. What is meant by distance?
Answers
Answer:
How far away the objects are from each other
if you are given pKa and pH and asked to find ratio of acid to base, what do you do?
Answers
The ratio of acid to base can be calculated using the Henderson–Hasselbalch equation.
To calculate the pH, the equation which represents an acid-base or a buffer solution is represented below is used.
pH = pKₐ + log([A⁻]/[HA])
Here HA is a weak acid and A- is as conjugate base.
One way to determine the pH of a buffer is by using the Henderson–Hasselbalch equation, which is
pH = pKₐ + log([A⁻]/[HA])
The pH of a substance or solution is referred to the degree of acidity or alkalinity of that substance. It is measured on a scale of 0 -14.
To learn more about pH check the link below-
https://brainly.com/question/26424076
#SPJ4
Fill in the blanks (Balancing chemical reactions)
BRAINLIEST
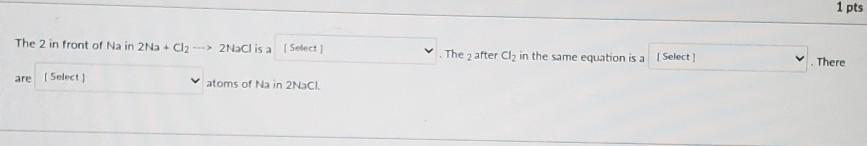
Answers
\( \huge{ \mathfrak{ \underline{ Answer \: \: ✓ }}}\)
The 2 in front of \(Na\) in \(2Na + Cl_2 \rightarrow 2NaCl \) is Coefficient.
_____________________________
The \( _2 \) after \( Cl_2\) in the same equation is sub script.
_____________________________
There are two atoms of \(Na\) in \(2NaCl\)
_____________________________
\(\mathrm{ \#TeeNForeveR}\)
In the harber-bosch process, the reactant nitrogen is drawn from the air while the hydrogen is produced by burning methane gas (CH4) in a series of processes that can be simplified as: CH4 + 2H2O —> CO2 + 4H2 3a. A small ammonia plant used 123,000 g of H2 gas per day. Determine the mass of CO2 (in g) that will be released as the H2 is produced. Show all work
Answers
Answer:Main Answer:
The mass of CO2 released in the production of 123,000 g of H2 gas is 265,200 g.
Explanation: From the given equation, we can see that the production of 4 moles of H2 requires the combustion of 1 mole of CH4, resulting in the release of 2 moles of H2O and 1 mole of CO2. The molar mass of H2 is 2 g/mol, and the molar mass of CO2 is 44 g/mol. Therefore, the mass of CO2 released in the production of 123,000 g of H2 is (123,000/2) x (1/4) x 44 = 265,200 g.
Bar magnets have a north pole and a south pole Latrisha places a bar magnet on three small straws, so it can roll. Her set up is shown below, placing which of the following objects at point X will cause the bar magnet to move away from point X.
Answers
The set up shown in the image indicates that the bar magnet is free to roll on three small straws. The magnet has a north pole and a south pole, and its movement is affected by the surrounding magnetic field. To make the bar magnet move away from point X, we need to place an object that will create a magnetic field that interacts with the magnet's field.
As we know, opposite poles attract, and similar poles repel. Therefore, placing a magnet with a north pole at point X will cause the bar magnet to move away from it as the north pole of the bar magnet is attracted to the south pole of the new magnet. Similarly, placing a magnet with a south pole at point X will cause the bar magnet to move away from it as the south pole of the bar magnet is attracted to the north pole of the new magnet.
However, if we place a magnet with the same pole as the bar magnet at point X, the bar magnet will move towards it due to the repulsion between similar poles. Therefore, to make the bar magnet move away from point X, we need to place a magnet with the opposite pole as the bar magnet at point X.
In conclusion, placing a magnet with a north pole at point X will cause the bar magnet to move away from it. Similarly, placing a magnet with a south pole at point X will also make the bar magnet move away from it. It is essential to remember that opposite poles attract, and similar poles repel when working with magnetic fields.
For more such question on indicates
https://brainly.com/question/29330415
#SPJ11
Note- The Question seems Incomplete, and complete question isn't available in the search engine.
indefinite shape, but definite volume
Answers
Answer:
A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. The change from solid to liquid usually does not significantly change the volume of a substance.
Explanation:
Select the curve that is produced by adding hydrochloric acid to 25 cm3 of sodium hydroxide.A,B,C or D

Answers
B
The sodium hydroxide (NaOH) solution is a basic solution, so the pH of that solution should be close to 14
then when adding hydrochloric acid (HCl) we start to neutralice the solution, meaning the pH must sift slowly to lower pH.
Assuming both solutions have similar concentration the pH shall shift form basic (above 7) to acid pH (below 7). Until now both B and D images agreed with the explanation given. To chose between them we need to remember that HCl is a very strong acid, which means that in solution will get to very acid solutions (very low pH values) which leaves only B as possible answer
1,3-Butadiene is a conjugated diene with the chemical formula C4H6true or false
Answers
It is true that 1,3-butadiene has the chemical formula C4H6 and is a conjugated diene. A popular monomer in the creation of synthetic rubber, polymers, and resins, it is a colourless, flammable gas.
A conjugated diene with the chemical formula C4H6 is 1,3-butadiene. A popular monomer in the creation of synthetic rubber, polymers, and resins, it is a colourless, flammable gas. Because of the butadiene's conjugated double bond system, pi-electrons are delocalized over the whole molecule, giving it special characteristics including high reactivity, a low boiling point, and a propensity for addition reactions. Butadiene is a crucial industrial chemical that is created by the catalytic dehydrogenation of butene or butane. It finds usage in a variety of petrochemical, polymer, and automotive applications.
learn more about chemical formula here:
https://brainly.com/question/29031056
#SPJ4
1. Which is conduction?
A. energy transfer from a reaction system to its surroundings
B. energy transfer as electromagnetic waves
C. energy transfer by currents of moving liquid or gas
D. energy transfer by direct contact
2. What is a chemical reaction system?
A. the reactants and products in a reaction
B. a diagram used to show energy changes in a reaction
C. everything outside the reaction
D. a container in which chemical reactions take place
3. In photosynthesis within one plant, other plants would be considered the _____
A. products.
B. system.
C. reactants.
D. surroundings.
4. The products of a particular reaction have more energy than the reactants. Which type of reaction has occurred?
A. convection
B. exothermic
C. radiation
D. endothermic
5. Which type of reaction is occurring during a fireworks show? How do you know?
A. It is an endothermic reaction. Fireworks are bright, indicating a release of light energy.
B. It is an exothermic reaction. Fireworks are bright, indicating a release of light energy.
C. It is an exothermic reaction. Heat must be added to light the fuses of the fireworks.
D. It is an endothermic reaction. Heat must be added to light the fuses of the fireworks.
PLZ HELP
Answers
Answer:
1. D
2. A
3. This question doesn't make sense
4. D
5. B or C
Explanation:
Answer: Which is conduction?
energy transfer by direct contact
What is a chemical reaction system?
the reactants and products in a reactionIn photosynthesis within one plant, other plants would be considered the ___
surrounding
The products of a particular reaction have more energy than the reactants. Which type of reaction has occurred?
endothermic
Which type of reaction is occurring during a fireworks show? How do you know?
It is an exothermic reaction. Fireworks are bright, indicating a release of light energy.
Explanation: just did the quick check got 100%
Which of the following statements regarding physical and chemical changes is true?
Answers
Halogens are active non metal
Answers
Answer:
True
Explanation:
They are actively non metals due to their electron configuration and number of valence electrons
PLEASE ANSWER!!!! 30 POINTS!!!!!!!
How many grams of NH3 form when 84 g N2 react completely?
3H2 + N2 ---> 2NH3
N2: 28 g/mol NH3: 17 g/mol
84 g N2 ---> g NH3
Answers
Answer: 84 g of N2 reacts completely to form 102 g of NH3.
Explanation: Change over the mass of N2 from grams to moles utilizing its molar mass:
84 g N2 × (1 mol N2 / 28 g N2) = 3 moles of N2
Utilize stoichiometry to calculate the number of moles of NH3 delivered, knowing that 3 moles of H2 are required to respond with 1 mole of N2:
3 moles of N2 × (2 moles of NH3 / 1 mole of N2) = 6 moles of NH3
Change over the number of moles of NH3 to grams utilizing its molar mass:
6 moles of NH3 × (17 g NH3 / 1 mol NH3) = 102 g NH3
what happens to the gaseous phase of water when it reaches a metal lid
Answers
Answer:
When the gaseous phase of water reaches a metal lid it condenses to form water.
Explanation:
The gaseous phase of water is at a higher temperature than the temperature of a metal lid. Thus when it comes in contact with the cold lid it loses heat and gets converted to the liquid phase.
When water vapor loses temperature, it undergoes a process called condensation, which results in its conversion to a liquid state. This process is essentially the opposite of vaporization. Typically, condensation occurs when a vapor is compressed or cooled to its saturation limit, causing the molecular density within the gas phase to reach its maximum threshold.
To know more about condensation,
https://brainly.com/question/30629848
TRUE OR FALSE : The aim of an experiment can often tell us which variable is the independent variable and which variable is the dependent variable
Answers
Answer:
True
Explanation:The whole point of and expirement is to test the indepedent variable and the outcom or what your measuring is the dependent
sorry for the tone its late where i live
Which substance is composed of only one type of atom? - Need asap!
Water
Gold
Salt
Sugar
Answers
Answer:
Gold
Hope it helps
have a good day