What do the conclusions tell about the experiment?
OA. The conclusions tell what other scientists think about the
experiment.
B. The conclusions tell how the experiment should be repeated.
C. The conclusions tell if the scientific method was followed.
D. The conclusions tell why the data support or reject the hypothesis.
Answers
The conclusions tell why the data support or reject the hypothesis. Option D
Conclusions drawn from an experiment provide an analysis and interpretation of the data collected during the experiment. They involve examining the results obtained and determining whether they align with the initial hypothesis or research question. The conclusions aim to provide a logical explanation based on the evidence gathered.
In scientific experiments, conclusions are drawn by analyzing the data in relation to the objectives of the study. The conclusions should indicate whether the data support or reject the hypothesis or research question that was initially proposed. This analysis helps to determine whether the experiment's findings align with the expected outcome.
Conclusions are essential in providing a summary of the experiment's results and offering explanations or interpretations based on the evidence collected. They play a crucial role in scientific research as they allow scientists to communicate their findings and contribute to the broader scientific community's understanding of a specific phenomenon or concept. Option D
For more such questions on hypothesis visit:
https://brainly.com/question/31293943
#SPJ8
Related Questions
Explain why Hydrogen-1 has a binding energy
of zero.
Answers
answers: therefore, binding energy equal to zero
Explanation:in this case of a hydrogen atom, it has only one proton and zero neutrons. in this case the proton is already seperated from other nucleons. so energy is not released in this case.
The weight of a Moon Rover on Earth is 6,000 newtons,
What is the approximate weight of a Moon Rover on the Moon?
A. O newtons
B. 600 newtons
O c. 1,000 newtons
D. 6,000 newtons
O E. 10,000 newtons
Answers
Answer: C. 1000 Newton
Explanation:
Given that :
Weight of moon rover on earth = 6000 newton
Approximate of moon rover on moon will be?
Weight of an object (W) = mass(m) × acceleration due to gravity(g)
To get the mass of moon rover(m) :
m = weight / acceleration due to gravity
g on earth = 9.8m/s²
Hence,
mass of moon rover = 6000/ 9.8
= 612.244kg
Surface gravity on the moon is about 1.6m/s²
Hence ;
Weight of moon rover on the moon :
Mass of moon rover × 1.6m/s²
= 612.244 × 1.6m/s²
= 979.59 Newton
= 1000 Newton (nearest thousand)
The approximate weight of the Moon Rover on the Moon is 1000 N
The correct answer to the question is Option C. 1000 N
We'll begin by calculating the mass of the Moon Rover. This can be obtained as follow:
Weight (W) = 6000 N
Acceleration due to gravity (g) = 9.8 m/s²
Mass (m) =?W = m × g
6000 = m × 9.8
m = 6000 / 9.8
m = 612.24 KgFinally, we shall determine the weight of the Moon Rover on the moon. This can be obtained as follow:Acceleration due to gravity of the moon (g) = 1.67 m/s²
Mass (m) = 612.24 Kg
Weight (W) =?W = m × g
W = 612.24 × 1.67
W ≈ 1000 NTherefore, the approximate weight of the Moon Rover on the Moon is 1000 N
The correct answer to the question is Option C. 1000 N
Learn more: https://brainly.com/question/19540959
A snack machine accepts only 5-centavo coins. Chocolate bars cost 25cent each,
packages of peanuts cost 75cent each and a can of cola costs 50 cent. How many 5-centavo
coins are needed to buy 2 chocolates bars, one pack of peanuts and a can of soda?
Answers
To buy two chocolate bars, one pack of peanuts, and a can of soda with a snack machine that only accepts 5-centavo coins, we need to Solve the Equation to calculate the total cost and the number of coins required. The answer to this question is 21 coins.
One chocolate bar costs 25 cent, so two chocolate bars cost 25 x 2 = 50 cent.One pack of peanuts costs 75 cent.A can of soda costs 50 cent.The total cost of these snacks is 50 + 75 + 50 = 175 cent.Now, we need to find how many 5-centavo coins make up 175 cent.1 centavo is equal to 0.05 cents.Therefore, 175 cent is equal to 175/0.05 = 3,500 centavos.
To find the number of 5-centavo coins required, we need to divide 3,500 by 5.3,500 ÷ 5 = 700 coins.So, it will take 700 5-centavo coins to buy two chocolate bars, one pack of peanuts, and a can of soda.
Learn more about equation visit :
brainly.com/question/14603452
#SPJ11
Beryllium (Be) and fluorine (F) combine to make the compound BeF2. What is the chemical name for this compound?
A.
beryllium fluoride
B.
beryllium fluorine
C.
beryllide fluoride
D.
beryllide fluorine
Answers
Answer:
The answer you're looking for is A
Explanation:
A is the answer
Explanation:
The two-way table shows the numbers of tropical cyclones that formed during the hurricane seasons over a 12-year period.
find the probability using the formula for conditional probability. express your first answer as a simplified fraction, and round your percent answer to the nearest tenth.
p(hurricane | northern hemisphere)
the probability is ____, or about ____%
show all work
Answers
The probability of a hurricane forming in the northern hemisphere is about 36.4%.
To find the probability of a hurricane forming in the northern hemisphere, we need to use the formula for conditional probability. The formula is P(A|B) = P(A and B) / P(B), where A and B are two events.
In this case, the events are "hurricane forming" and "in the northern hemisphere". We can fill in the formula with the values from the two-way table:
P(hurricane | northern hemisphere) = P(hurricane and northern hemisphere) / P(northern hemisphere)
From the table, we see that there were 43 hurricanes that formed in the northern hemisphere. The total number of storms in the northern hemisphere is 75+43 = 118. Therefore:
P(hurricane | northern hemisphere) = 43/118
We can simplify this fraction by dividing the numerator and denominator by the greatest common factor of 43 and 118, which is 1. So:
P(hurricane | northern hemisphere) = 43/118 ≈ 0.3644
To express this as a percentage, we multiply by 100 and round to the nearest tenth:
P(hurricane | northern hemisphere) ≈ 36.4%
To know more about probability visit:
https://brainly.com/question/31828911
#SPJ11
What is the overall enthalpy change dhrxn for the system? -1,300 kj -300 kj 300 kj 1,300 kj
Answers
The overall enthalpy change i.e ΔH for the system is -1300 kJ.
Enthalpy change in a reaction is defined as the difference in the potential energy of the products and the potential energy of the reactants.
This is represented by ΔHreaction.
The reaction of enthalpy change is given as
ΔHreaction= ΔHproducts - ΔHreactants
where ΔHproducts is the potential energy of the products
=[(-200) + (-300)]kJ
= -500kJ
And, ΔHreactants is the potential energy of the reactants
=800KJ
Therefore, by putting these values ΔHreaction can be calculated as
ΔHreaction
= [-500-800] KJ
=-1300 KJ
Thus, the overall enthalpy change is -1300KJ
To know more about enthalpy change here
https://brainly.com/question/12200084
#SPJ4
How is anaerobic respiration helpful to you as and individual or society please answer I really need help with this don’t answer for points because I will report!!
Answers
Answer:
Anaerobic exercise uses energy that’s readily available in your muscles, because the body isn’t relying on oxygen, these strong, powerful movements can only be sustained for 10 to 15 seconds.
Explanation:
Anaerobic exercise is beneficial for good health because it:
Strengthens bones
Burns fat
Builds muscle
Maintains muscle mass, which is important for people as they age
As we age, we tend to lose muscle at a rate of about 1 percent per year after age 27, unless we actively work to slow that decline, Muscle is a metabolically active tissue and the more of it we can maintain, the more calories we can burn while at rest.
Anaerobic respiration occurs more quickly than aerobic respiration.
A major advantage of aerobic respiration is the amount of energy it releases. Without oxygen, organisms can split glucose into just two molecules of pyruvate. This releases only enough energy to make two ATP molecules.
Methanogenesis, a form of carbon-dioxide respiration, that is used to produce methane gas by anaerobic digestion. Biogenic methane is used as a sustainable alternative to fossil fuels.
Anaerobic respiration is a critical component of the global nitrogen, iron, sulfur, and carbon cycles through the reduction of the oxyanions of nitrogen, sulfur, and carbon to more-reduced compounds.
Specific types of anaerobic respiration are also critical in bioremediation, which uses microorganisms to convert toxic chemicals into less-harmful molecules to clean up contaminated beaches, aquifers, lakes, and oceans. For example, toxic arsenate or selenate can be reduced to less toxic compounds by various anaerobic bacteria via anaerobic respiration.
Hope you will find it helpful. I tried my best. Thank you.
What’s the chemical name of
NaCl + F2 = NaF + Cl2
(Or 2NaCl + F2 = 2 NaF + Cl2)
Answers
Answer:
sodium chloride+difluorine=sodium fluoride+dichlorine
Explanation:
these are the elements name
Which scientist did people think was insane so they dismissed his ideas for almost 2000 years?
Answers
I think let me know if I am wrong
Az Which of the following pairs of molecules could be joined together by peptide bond in a dehydration reaction? and 3 12 and 13 and 7 8 and 9
Answers
The pairs of molecules that could be joined together by a peptide bond in a dehydration reaction are 3 and 12, and 13 and 7. Option A, and B are correct.
The peptide bond is formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH₂) of another amino acid, with the loss of a water molecule. So, in order for two molecules to be joined together by a peptide bond in a dehydration reaction, one molecule should have a carboxyl group and the other should have an amino group.
Option 3 and 12: molecule 3 is glycine, which has an amino group (-NH₂) and molecule 12 is glutamic acid, which has a carboxyl group (-COOH). So, they could be joined together by a peptide bond in a dehydration reaction.
Option 13 and 7: molecule 13 is proline, which has an amino group (-NH₂) and molecule 7 is aspartic acid, which has a carboxyl group (-COOH). So, they could be joined together by a peptide bond in a dehydration reaction.
Option 8 and 9: both molecules 8 and 9 are nucleotides and do not contain amino or carboxyl groups. So, they cannot be joined together by the peptide bond in dehydration reaction.
Hence, A. B. 3 and 12, 13 and 7 is the correct option.
To know more about dehydration reaction here
https://brainly.com/question/13448613
#SPJ4
--The given question is incomplete, the complete question question is
"Az Which of the following pairs of molecules could be joined together by peptide bond in a dehydration reaction? A) 3 and 12 B) 13 and 7 C) 8 and 9."--
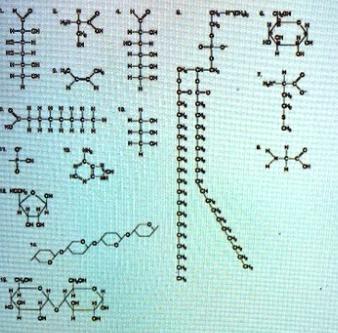
HELP I’ll give Brainliest

Answers
Answer: The first question’s answer is 2 and the second question answer is 3. 12.B, 13.C
Explanation:
A mixture of two hydrocarbons, C8H18 (octane) and C7H8 (toluene), has a mass of 161 g. The hydrocarbon mixture is burned in excess oxygen to form a mixture of carbon dioxide and water that contains 1.52 times as many moles of carbon dioxide as water. Find the masses of C8H18 and C7H8 in the mixture.
Answers
The solution is the mass of C8H18 is 69 grams and the mass of C7H8 is 92 grams.
Given: The hydrocarbon mixture contains two hydrocarbons - C8H18 and C7H8.Mass of the mixture = 161 g.Mixtures of two hydrocarbons, C8H18 (octane) and C7H8 (toluene), are burned in excess oxygen to form a mixture of carbon dioxide and water that contains 1.52 times as many moles of carbon dioxide as water. We need to calculate the masses of C8H18 and C7H8 in the mixture.
To calculate the mass of the mixture:Let the mass of C8H18 in the mixture = x gramsTherefore, the mass of C7H8 in the mixture = (161 - x) grams.Then, calculate the number of moles of CO2 and H2O produced.
Using stoichiometry,2 C8H18 + 25 O2 ⟶ 16 CO2 + 18 H2O(2 × moles of C8H18) + (25 × moles of O2) ⟶ (16 × moles of CO2) + (18 × moles of H2O)As per the given condition,Number of moles of CO2 = 1.52 × number of moles of H2OMass of CO2 = Moles of CO2 × Molar mass of CO2 = 1.52 × Moles of H2O × Molar mass of CO2 = 1.52 × (18 × 2 × Moles of C8H18) × 44/22 = 132 × Moles of C8H18Mass of H2O = Moles of H2O × Molar mass of H2O = (18 × 2 × Moles of C8H18) × 18/1000 = 0.648 × Moles of C8H18Therefore, we have the following equation:Mass of C8H18 + Mass of C7H8 = 161 grams => x + (161 - x) = 161 grams=> x = 69 grams => Mass of C8H18 = 69 g.Mass of C7H8 = (161 - 69) g = 92 g.
Thus, the masses of C8H18 and C7H8 in the mixture are 69 g and 92 g, respectively. Hence, the solution is the mass of C8H18 is 69 grams and the mass of C7H8 is 92 grams.
Learn more about Carbon dioxide here,Carbon dioxide is a naturally occurring gas that is vital to plant and animal life, yet carbon dioxide emissions from bu...
https://brainly.com/question/22963529Carbon dioxide is a naturally occurring gas that is vital to plant and animal life, yet carbon dioxide emissions from bu...
https://brainly.com/question/22963529
#SPJ11
Can someone please help !! I just need someone to help me figure out how to solve it and solve the picture as an example

Answers
The molar concentration of Al(OH)₃ in the solution is 1.61 M.
we need to calculate the number of moles of Al(OH)3 in the solution:
Number of moles of Al(OH)₃ = mass of Al(OH)3 / molar mass of Al(OH)3
Molar mass of Al(OH)₃ = (1 x atomic mass of Al) + (3 x atomic mass of O) + (3 x atomic mass of H)
Molar mass of Al(OH)₃ = (1 x 26.98 g/mol) + (3 x 16.00 g/mol) + (3 x 1.01 g/mol) = 78.00 g/mol
Number of moles of Al(OH)₃ = 62.7 g / 78.00 g/mol = 0.804 moles
Next, we need to calculate the volume of the solution in liters:
Volume of solution = 500.0 mL = 500.0 mL x (1 L/1000 mL) = 0.500 L
Finally, we can calculate the molar concentration of Al(OH)₃
Molarity = moles of solute/volume of solution in liters
Molarity = 0.804 moles / 0.500 L = 1.61 M
Therefore, the molar concentration of Al(OH)₃ in the solution is 1.61 M.
learn more about Molar Mass here
https://brainly.com/question/837939
#SPJ1
The rate constant for a reaction at 25.0 degrees Celsius is 0.010 s-1 and its activation energy is 35.8 kJ. Find the rate constant at 50.0 degrees Celsius.
Answers
A proportionality constant that represents the link between the molar concentration of reactants and the rate of a chemical reaction is denoted by the letter "k," which stands for the rate constant.
To find the rate constant at 50 °C for a reaction with a rate constant of 0.010 s-1 and an activation energy of 35.8 kJ, we will use the Arrhenius equation.
What is the Arrhenius equation?
The Arrhenius equation is an equation that relates the rate constant of a chemical reaction to temperature and activation energy.
Determining the rate constant:
The equation is written as: k = Ae^(-Ea/RT)
Where:k is the rate constant
A is the pre-exponential factor or frequency factorEa is the activation energyR is the gas constantT is the absolute temperature in Kelvin
R = 8.3145 J/Kmol
Converting the activation energy to J/mol:35.8 kJ/mol x 1000 J/kJ = 35,800 J/mol
Given:
T1 = 25°C = 298 K (Kelvin)T2 = 50°C = 323 K (Kelvin)Ea = 35,800 J/molk1 = 0.010 s^-1
Using the Arrhenius equation:
k2/k1 = e^(-Ea/R) [(1/T2) - (1/T1)]k2/0.010 s^-1 = e^(-35,800 J/mol / 8.3145 J/Kmol) [(1/323 K) - (1/298 K)]k2 = 0.010 s^-1 x e^(12,717.1 J/mol / 8.3145 J/Kmol) [0.0033 K^-1]k2 = 0.087 s^-1 (3 significant figures)
Therefore, the rate constant at 50°C is 0.087 s-1.
To know more about the activation energy https://brainly.com/question/28384644
#SPJ11
Please answer quick!!
Which statement best explains how sexual reproduction increases variation within a species?
A. It causes the genes of two different individuals to mix.
B. It changes the base sequence of an organism's DNA.
C. It changes the trait that a given gene produces.
D. It copies one parent's genes to make each offspring.
Answers
Answer:
A
Explanation:
it causes the genes of two different individuals to mix
Answer:
Explanation:
A. It causes the genes of two different individuals to mix.
Select the step(s) that will compose a rationale for the cation Ag being absent in an unknown (but Pbº is present). Select one or more: a. A yellow precipitate did not form when KyCrO was added in step 1-C. b. The white solid did not turn black upon addition of NaOH and SnCl2. c. The presence of a light blue decantate in step 1-A. d. All of the white precipitate from step 1-A dissolved in hot water. e. A lack of dark blue colored solution after addition of 15 M NH 3. f. A white precipitate did not form in step 2-B. g. A reddish brown precipitate did not form after adding K Fe(CN)6- No white precipitate formed when 6 M HCI was added to the unknown solution in step 1.A. h. The white precipitate from step 1-B dissolved in 6 M NH3 and then reformed when 6 M HNO, was added.
Answers
The steps that can be used as a rationale for the absence of Ag in an unknown solution (but with Pb²⁺ present) in a chemical analysis experiment are: a, b, d, e, and h.
Steps (a), (b), (d), (e), (f), and (g) can be used as a rationale for the absence of Ag in an unknown solution with Pb²⁺ present in a chemical analysis experiment. The lack of yellow precipitate with KyCrO, no blackening of the white solid with NaOH and SnCl₂, and the absence of a white precipitate with 6M HCl suggest the absence of Ag.
The presence of a light blue decantate in step 1-A and the lack of dark blue solution after the addition of 15M NH₃ further support this conclusion. Additionally, the absence of a white precipitate in step 2-B and the lack of reddish-brown precipitate after adding KFe(CN)₆⁻ also indicate the absence of Ag in the unknown solution.
To know more about precipitate, here
brainly.com/question/31046678
#SPJ4
--The complete question is, Which step(s) can be used as a rationale for the absence of Ag in an unknown solution (but with Pb²⁺ present) in a chemical analysis experiment? Select all that apply:
a. A yellow precipitate did not form when KyCrO was added in step 1-C.
b. The white solid did not turn black upon addition of NaOH and SnCl2.
c. The presence of a light blue decantate in step 1-A.
d. All of the white precipitate from step 1-A dissolved in hot water.
e. A lack of dark blue colored solution after addition of 15 M NH3.
f. A white precipitate did not form in step 2-B.
g. A reddish brown precipitate did not form after adding K Fe(CN)6-
h. The white precipitate from step 1-B dissolved in 6 M NH3 and then reformed when 6 M HNO3 was added.
Note: The question is referring to a specific chemical analysis experiment, where the absence of Ag needs to be justified in an unknown solution with the presence of Pb²⁺.--
Polonium-218 undergoes beta decay, converting a neutron into a proton. Then the daughter isotope undergoes another beta decay, again converting a neutron into a proton. Which equation correctly describes this process? Use the periodic table link from the tools bar to answer the question.




Answers
Answer: \(^{218}_{84}Po \rightarrow ^{218}_{85}At + ^{0}_{-1}e\) is the correct equation for beta decay.
Explanation:
When a beta particle, that is, \(^{0}_{-1}e\) is emitted in a radioactive decay then it is known as beta decay.
Therefore, beta decay of Polonium-218 is as follows.
\(^{218}_{84}Po \rightarrow ^{218}_{85}At + ^{0}_{-1}e\)
Therefore, we can conclude that \(^{218}_{84}Po \rightarrow ^{218}_{85}At + ^{0}_{-1}e\) is the correct equation for the given beta decay.
Answer:
A
Explanation:
The top dudes answer summed up.
what is a galvanic cell made of?
A. two electrodes in electrolyte solution
B. two beakers connected by a tube
C. two ceramic plates in pure water
D.two medal strips surrounded by air
Answers
Explanation:
A because A galvanic cell consists of two half-cells, such that the electrode of one half-cell is composed of metal A, and the electrode of the other half-cell is composed of metal B; the redox reactions for the two separate half-cells are thus: An+ + ne− ⇌ A.
Answer:
A
Explanation:
The first excited vibrational energy level of diatomic chlorine (Cl2) is 558 cm−1 above the ground state. Wavenumbers, the units in which vibrational frequencies are usually recorded, are effectively units of energy, with 1 cm−1=1.986445∗10−23 J. A. If every vibrational energy level is equally spaced, and has a degeneracy g, of 1 , sum over the lowest 4 vibrational levels to obtain a vibrational partition function Q, for chlorine at 25∘C. Your answers will be as sum of exponentials, simplify them as much as you can. B. Let the N1 and N2 be the population of chlorine molecules in the first and second excited vibrational energy levels respectively. Find the relative population between the excited states N1N2, at 298 K(25∘C) [Convert energy into Joules first before finding the exponentials for the partition function. See practice problem set 5 . The ground state is at 0 J energy level. The Boltzmann constant, kB= 1.38065×10−23 J]
Answers
The vibrational partition function (Q) for chlorine at 25°C, summing over the lowest 4 vibrational levels, is given by the simplified expression: Q = e^(-2.220).
The relative population between the first and second excited vibrational energy levels (N1/N2) of chlorine at 298 K (25°C) can be calculated using the relative Boltzmann factors, taking into account the energies of the levels and the Boltzmann constant.
The vibrational partition function (Q) represents the sum of the Boltzmann factors for all the vibrational energy levels. For a diatomic molecule like chlorine (Cl2), assuming equally spaced vibrational energy levels and a degeneracy (g) of 1 for each level, we can calculate the partition function.
To calculate Q, we sum over the lowest 4 vibrational levels, taking into account the energy spacing between levels.
The energy spacing between levels is given as 558 cm^(-1), which we convert to Joules using the conversion factor of 1 cm^(-1) = 1.986445 × 10^(-23) J.
Using the formula for the partition function:
Q = e^(-E1/(kT)) + e^(-E2/(kT)) + e^(-E3/(kT)) + e^(-E4/(kT))
Substituting the values:
Q = e^(-5581.98644510^(-23)/(1.3806510^(-23)(25+273)))
Simplifying the exponent and performing the calculations:
Q ≈ e^(-2.220)
Therefore, the vibrational partition function (Q) for chlorine at 25°C, summing over the lowest 4 vibrational levels, is approximately e^(-2.220).
The relative population between the first and second excited vibrational energy levels (N1/N2) of chlorine at 298 K (25°C) can be calculated using the relative Boltzmann factors, taking into account the energies of the levels and the Boltzmann constant.
The relative population (N1/N2) between two vibrational energy levels can be determined using the Boltzmann factors, which depend on the energies of the levels and the temperature.
The energy difference between the ground state and the first excited level is given as 558 cm^(-1), which we convert to Joules using the conversion factor of 1 cm^(-1) = 1.986445 × 10^(-23) J.
Using the Boltzmann factor formula:
N1/N2 = e^(-ΔE/(k*T))
Substituting the values:
N1/N2 = e^(-5581.98644510^(-23)/(1.38065*10^(-23)*298))
Simplifying the exponent and performing the calculations:
N1/N2 ≈ e^(-1.524)
Therefore, the relative population between the first and second excited vibrational energy levels (N1/N2) of chlorine at 298 K (25°C) is approximately e^(-1.524).
Note: The relative population is given as a ratio of the populations between the two levels.
Learn more about Boltzmann factors here: brainly.com/question/30763196
#SPJ11
PLEASEEE HELP MEEEE!!! How many grams of iron (III) oxide will be produced if 4300 kJ of heat energy is released?
4 Fe+ 3 O2 → 2 Fe2O3
ΔH = -1652 kJ
Answers
Answer: 652.8 g of iron (III) oxide produced.
Explanation:
To calculate the amount of iron (III) oxide produced, we use the enthalpy change of the reaction to determine the amount of energy released and convert it to moles of Fe2O3 produced. Then, we multiply by the molar mass of Fe2O3 to obtain the mass of Fe2O3 produced. Using these calculations, we get 652.8 g of iron (III) oxide produced.
580.84 grams of iron (III) oxide will be produced when 4300 kJ of heat energy is released.
Given:
Enthalpy change (∆H) value: ∆H = -1652 kJ
Amount of heat energy released: 4300 kJ
From the balanced equation:
4Fe + 3O₂ → 2 Fe₂O₃
The molar ratio between Fe₂O₃ and ∆H is 2:1652 kJ.
To find the molar amount of Fe₂O₃ produced, the following calculation:
\(4300 \times \frac{2}{1652}\) = 5.20 mol Fe₂O₃
To convert this into grams, it is required to multiply the molar amount by the molar mass of Fe₂O₃:
5.20 × 2 × 55.85 = 580.84 g
Therefore, 580.84 grams of iron (III) oxide will be produced when 4300 kJ of heat energy is released.
Learn more about heat energy, here:
https://brainly.com/question/1495272
#SPJ2
True/False: Solubility is the ease at which a substance burns.
Answers
Answer:
False
Explanation:
Solubility refers to the ability of a substance to dissolve in a solvent, usually a liquid. It is a measure of the amount of a substance that can dissolve in a given amount of solvent at a given temperature. Solubility is not related to the ability of a substance to burn.
By adding electrons to an uncharged object, the object *
becomes negatively charged
becomes positively charged
becomes energized
changes color
Answers
Answer:
becomes negatively charged
Explanation:
this is because when an object receives electrons it becomes negatively charged and the object that loses electrons becomes positively charged.
Answer:
First one because by adding more electrons it makes something more negative. So it becomes negatively charged
In an electron configuration ,what does the s in 1s2 represent?
Answers
Answer:
The superscript after the letter
tells us the electrons are in the n=2 energy level.
Explanation:
As a gaseous element condenses, the atoms become ________ and they have ________ attraction for one another.
Answers
As a gaseous element condenses, the atoms become closer together and they have more attraction for one another.
What is Condensation?The transformation of water vapour into liquid is known as condensation. The process is the opposite of evaporation, in which liquid water turns into a vapour. Either the air is chilled to its dew point or it gets too saturated with water vapour to retain any more water, causing condensation to occur.
Attraction - Gas Particles move continuously in a straight path in a gas. They are significantly further apart and move independently of one another because the kinetic energy of the molecule is larger than the attraction force between them. There are often almost no attractive forces between particles.
to learn more about condensation go to - https://brainly.com/question/1268537
#SPJ4
look below for question

Answers
Please help me!!!
what is the density of a sphere that on earth weighs 4000 g and has a diameter of 150?
Answers
Answer:
The answer is 0.002 g/cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass of sphere = 4000 g
Volume of a sphere is given by
\(V = \frac{4}{3} \pi {r}^{3} \\ \)
where r is the radius
\(r = \frac{diameter}{2} \\ \)
From the question
diameter = 150 cm
\(r = \frac{150}{2} = 75 \: \: \: cm\)
The volume of the sphere is
\(V = \frac{4}{3} \times {75}^{3} \pi \\ = \frac{4}{3} \times 421875\pi \\ = 562500\pi \\ = 1767145.86...\)
We have the answer as
volume = 1767145.86 cm³
So we have
\(density = \frac{4000}{1767145.86} \\ = 0.002263536...\)
We have the final answer as
0.002 g/cm³Hope this helps you
Please help, I need the answer asap. Got a test on Monday!!

Answers
1. The bacterial cell shown is a prokaryotic cell.
2a. The similarities between prokaryotic and eukaryotic cells include:
they both have cell membranesthey both carry out the activities of lifethey both contain genetic material in the form of DNA2b. The differences between prokaryotic and eukaryotic cells include:
eukaryotic cells have membrane-bound organelles but prokaryotic cells do noteukaryotic cells have a membrane-bound nucleus but prokaryotic cells do notWhat are eukaryotic and prokaryotic cells?Eukaryotic cells are cells that possess a membrane-bound nucleus as well as membrane-bound cell organelles.
Eukaryotic cells are cells of higher organisms such as animals and plants.
Prokaryotic cells are cells that lack a membrane-bound nucleus as well as membrane-bound cell organelles.
Prokaryotic cells are cells of lower organisms such as bacteria.
Learn more about eukaryotic and prokaryotic cells at: https://brainly.com/question/15842891
#SPJ1
The Liquified Petroleum Gas (LPG) has the composition of 60% Propane (C 3
H 8
) and 40% Butane (C 4
H 10
) by volume: (a) Find the wet volumetric and gravimetric analysis of the products of combustion when the equivalence ratio (Φ)=1.0. (b) What is the stoichiometric air to fuel ratio for the LPG.
Answers
The balanced combustion reaction for propane can be represented as:
C₃H₈ + (5/2)O₂ → 3CO₂ + 4H₂O
And the balanced combustion reaction for butane can be represented as:
C₄H₁₀ + (6.5)O₂ → 4CO₂ + 5H₂O
Since LPG is composed of 60% propane and 40% butane by volume, we can calculate the wet volumetric and gravimetric analysis based on these proportions.
Wet volumetric analysis:
For the wet volumetric analysis, we consider the volume of the products of combustion relative to the volume of the LPG consumed.
Propane (C₃H₈):
The stoichiometric coefficient of propane in the combustion reaction is 3. Therefore, for every mole of propane burned, we will have 3 moles of CO₂ and 4 moles of H₂O formed.
Butane (C₄H₁₀):
The stoichiometric coefficient of butane in the combustion reaction is 4. Therefore, for every mole of butane burned, we will have 4 moles of CO₂ and 5 moles of H₂O formed.
Considering the initial composition of 60% propane and 40% butane by volume, we can calculate the volumetric composition of the products of combustion:
Volumetric composition of CO₂:
(0.6 * 3) + (0.4 * 4) = 3.6
Volumetric composition of H₂O:
(0.6 * 4) + (0.4 * 5) = 4.6
Therefore, the wet volumetric analysis of the products of combustion is 3.6 parts CO₂ to 4.6 parts H₂O.
Wet gravimetric analysis:
For the wet gravimetric analysis, we consider the mass of the products of combustion relative to the mass of the LPG consumed.
Using the molar masses of the compounds involved in the combustion reaction:
Molar mass of CO₂ = 44 g/mol
Molar mass of H₂O = 18 g/mol
Gravimetric composition of CO₂:
(0.6 * 3 * 44 g/mol) + (0.4 * 4 * 44 g/mol) = 158.4 g
Gravimetric composition of H₂O:
(0.6 * 4 * 18 g/mol) + (0.4 * 5 * 18 g/mol) = 74.4 g
Therefore, the wet gravimetric analysis of the products of combustion is 158.4 grams CO₂ to 74.4 grams H₂O.
(b) The stoichiometric air to fuel ratio for LPG can be determined based on the balanced combustion equations for propane and butane.
For propane (C₃H₈):
C₃H₈ + (5/2)O₂ → 3CO₂ + 4H₂O
The stoichiometric coefficient for propane is 1, which means we need 5/2 moles of O₂ for every mole of propane.
For butane (C₄H₁₀):
C₄H₁₀ + (6.5)O₂ → 4CO₂ + 5H₂O
Some viruses attack cells by attaching to their outer covering entering and taking over their genetic machinery.viruses are able to invade cells after first attaching to their
Answers
In an _________________ reaction, heat is absorbed and the temperature of the system decreases.
Exothermic
Endothermic
Endoscopic
Exoskeleton
Answers
Answer:
Endothermic , this is correct answer