what do I do when my dog ate my secret 10 pound stash of chocolate and he won't move please help what do i do oh god help me pls
Answers
Answer:
call an vet ASAP 10 pounds of chocolate is very bad for a dog.
Explanation:
Chocolate is one of the worst things a dog can eat, and 10 pounds of it is worst! Take your dog to the vet IMMEDIATELY! I hope that that the dog will survive.
-------------------------------------------------------------------------------------------------------------Brainiest would make my day, but you don’t have to give it! You’re welcome.
Related Questions
3. Sulfur does not dissolve in water, but it does dissolve in carbon disulfide potassium nitrate fails to dissolve in carbon disulfide, but it dissolves in water; and carbon does not dissolve in either of these liquids. Using the above date, outline a process for separating these three components of gunpowder
Answers
The components of the gunpowder could be separated by dissolution in water and carbon disulfide.
What is gunpowder?The gunpowder is a mixture that is used in guns and some other explosives. The gun powder is a mixture of substances. The substances that are found in gun powder are; potassium nitrate, charcoal and sulfur.
Now, we could first add water to the system. The water would remove the potassium nitrate from the mixture then we can filter. After the filtration, carbon disulfide could be added to remove sulfur. The residue that is obtained now contains the carbon.
Learn more about gunpowder mixture:https://brainly.com/question/2289235
#SPJ1
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
phyy S. Use different flowers to make a variety of dyes and try to market the dyes. State the properties of the dyes made.
Answers
The properties of the dyes you can make from different flowers are:
Color VariationNatural and Eco-friendlyAromatic QualitiesLightfastness and DurabilityNatural VariabilityWhat is the variety of dyesFlower dyes have unique colors to offer a range of options for marketing. Rose petals yield pink and red shades. They are Natural and safe. Eco-conscious consumers prefer synthetic-free products, making your dyes attractive.
In terms of Aromatic Qualities: Lavender and jasmine smell nice. Using these flowers in dyes adds subtle scents for a sensory experience. Lightfastness and durability are crucial for creating dyes that resist fading when in the sunlight.
Learn more about variety of dyes from
https://brainly.com/question/30638068
#SPJ1
125 cm to m
I am trying to find what 125 centimeters would be in meters
Answers
Answer:
The answer is 1.25m
How many protons are pumped per NADH molecules in electron transport chain?
Answers
In the electron transport chain, 1 NADH molecule pumps 10 protons.
The electron transport chain (ETC) is a series of protein complexes that transfer electrons from NADH and FADH2 to oxygen. As electrons are transferred, energy is released, which is used to pump protons (H+) from the mitochondrial matrix to the intermembrane space. This creates a proton gradient, which drives the synthesis of ATP by ATP synthase.
Each NADH molecule that enters the ETC pumps 10 protons across the inner mitochondrial membrane. This is because four protons are pumped by complex I, four protons are pumped by complex III, and two protons are pumped by complex IV.
The number of ATP molecules produced per NADH and FADH2 molecule varies depending on the organism. In humans, each NADH molecule produces about 3 ATP molecules, while each FADH2 molecule produces about 2 ATP molecules.
Thus, in the electron transport chain, 1 NADH molecule pumps 10 protons.
To learn more about NADH :
https://brainly.com/question/11538586
#SPJ11
2. What happens to the temperature of air when it is compressed?
Answers
Explanation:
the pressure and temperature of the air increase
reason:
the volume of the space containing air decreases.
Explanation:
Whether you know it or not, compressed air is involved in every aspect of our lives, from the balloons at your birthday party to the air in the tires of our cars and bicycles. It was probably even used when making the phone, tablet or computer you’re viewing this on.
The main ingredient of compressed air is - you guessed it! - air. Air is a gas mixture, which means it consists of many different gases. Primarily these are nitrogen (78%) and oxygen (21%).
The temperature of the air is directly proportional to the mean kinetic energy of these molecules. This means that the air temperature will be high if the mean kinetic energy is large (and the air molecules move faster). The temperature will be low when the kinetic energy is small.
Compressing the air makes the molecules move more rapidly, which increases the temperature. This phenomenon is called “heat of compression”. Compressing air is literally to force it into a smaller space and as a result bringing the molecules closer to each other. The energy that gets released when doing this is equal to the energy required to force the air into the smaller space. In other words, it stores the energy for future use.
Complete the sentence!
Organisms in an ecosystem compete for _____. (Select all that apply).
A : shelter
B : water
C : food
D : space
Answers
Answer:
I personally think it would be all of the above
What type of glass has been exposed to high temperatures, so that when it breaks, it shatters into tiny pebble-like fragments that are less dangerous?
Answers
The type of glass that has been exposed to high temperatures and shatters into tiny pebble-like fragments when it breaks is called tempered glass.
Tempered glass is a type of safety glass that has been treated with heat and chemicals to make it stronger and more durable than regular glass. When it breaks, it shatters into small, rounded fragments that are less likely to cause injury than the sharp shards produced by regular glass. Tempered glass is commonly used in applications where safety is a concern, such as car windows, shower doors, and storefront windows. It is also used in the construction of buildings, furniture, and appliances.
To know more about temperature
brainly.com/question/11464844
#SPJ4
Why is formula equation more meaningful than a word equation?
Answers
The formula equation is more meaningful than a word equation because the word equation only defines what the reactants and products are, whereas the formula equation defines which and how much of each element is present in the chemicals and also give some indication as to their structure.
Answer:
The formula equation is more meaningful than a word equation because the word equation only defines what the reactants and products are, whereas the formula equation defines which and how much of each element is present in the chemicals and also give some indication as to their structure
Explanation:
The formula equation is more meaningful than a word equation because the word equation only defines what the reactants and products are, whereas the formula equation defines which and how much of each element is present in the chemicals and also give some indication as to their structure
The center of the atom is known as the?
Answers
Answer:
nucleus
Explanation:
An atom itself is made up of three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons. The protons and the neutrons make up the center of the atom called the nucleus and the electrons fly around above the nucleus in a small cloud.
hope that helps!
is the positively charged center of the atom consisting of protons and neutrons.
If energy was added to solid, what state would it change to
A. Super solid
B. Plasma
C. Liquid
Answers
Answer:
Explanation:
The answer is C Liquid
3. Answer the following two questions (20 points each part is 10 points) a. The orthoclose (potassium feldspar) clay mineral reacts with the HF/HCL mixture according to the following stochiometric reaction equation. For the 3 wt % HF (specific gravity of about 1.152 and MW=20) reacting with orthoclase feldspar (MW = 278.4 and p = 2.65 gr/cc) you are asked to calculate the gravimetric and volumetric dissolving powers Orthoclase (potassium feldspar): KAISI 308 + 14HF + 2H+K+ + AIF + 3SiF4 + 8H₂O b. A sandstone with a porosity of 0.22 containing 12% (volume) calcite (CaCO3) is to be acidized. If the HCI preflush is to remove all carbonates 36 inches beyond a 0.328-ft radius wellbore before the HF/HC1 stage enters the formationbefore the HF/HC1 stage enters the formation, what minimum preflush volume (gallons of acid solution per foot of formation thickness) is required if the preflush is 15% HCl solution?
Answers
The minimum preflush volume (gallons of acid solution per foot of formation thickness) required is:Volume of preflush solution (gallons/ft) = 0.17045 x 33.45= 5.7 gallons/ft.
a. Dissolving power of HF/HCL mixture:For the given equation, the molecular weight of potassium feldspar is 278.4 and the specific gravity of HF (3% solution) is 1.152. Therefore, we can calculate the gravimetric dissolving power of HF/HCl mixture as follows:Weight of HF = 3/100 x 1 x 1000 = 30 g/LiterThe equation requires 14 moles of HF to dissolve 1 mole of orthoclase feldspar. Therefore, the number of moles of HF required to dissolve 3% of orthoclase feldspar is:(14/1) x (3/100) = 0.42 mole/Liter
The volume of HF required to dissolve 3% of orthoclase feldspar is therefore:Volume of HF = (0.42 x 20)/30 = 0.28 L/LiterThe gravimetric dissolving power of HF/HCl mixture is calculated as follows:Dissolving power = (MW of orthoclase feldspar)/(Volume of HF required to dissolve 3% orthoclase feldspar)Dissolving power = 278.4/0.28 = 994.28 g/Liter
The volumetric dissolving power of HF/HCl mixture is calculated as follows:Dissolving power = (MW of orthoclase feldspar)/(Number of moles of HF required to dissolve 3% orthoclase feldspar)Dissolving power = 278.4/(0.42 x 20) = 330.86 g/Literb. Minimum preflush volume (gallons of acid solution per foot of formation thickness) required:Given data:Porosity = 0.22Volume of calcite = 12%Volume of sandstone = 88%Volumetric ratio of acid to sandstone (S):A = 1 - 0.12 = 0.88B = 0.12S = 0.15/0.88 = 0.17045The radius of the wellbore (r) is 0.328 ft.The volume of the annular region that needs to be flushed = πr²h= 3.14 x 0.328² x 36= 12.61 cubic feetVolume of the sandstone = Volume of the annular region that needs to be flushed/porosity= 12.61/0.22= 57.32 cubic feetThe thickness of the sandstone layer (h) = Volume of sandstone/area of annular region that needs to be flushed= 57.32/(π(0.328)² - π(0.328-0.0625)²)= 33.45 ft
Therefore, the minimum preflush volume (gallons of acid solution per foot of formation thickness) required is:Volume of preflush solution (gallons/ft) = 0.17045 x 33.45= 5.7 gallons/ft.
To learn more about Volume visit;
https://brainly.com/question/28058531
#SPJ11
The hydra is an organism that reproduces by growing another organism from the sie of its tubular body. What type of asexual reproduction is this?
Answers
Answer:
Explanation:
budding
Figure 13.3 (a) Hydra reproduce asexually through budding: a bud forms on the tubular body of an adult hydra, develops a mouth and tentacles, and then detaches from its parent. The new hydra is fully developed and will find its own location for attachment.
The activation energy of a reaction is 55.8 kJ/mol and the frequency factor is 1.5×10^11/s.
Calculate the rate constant of the reaction at 24 ∘C
Answers
Answer:
23.0 s⁻¹ is rate constant
Explanation:
Using the Arrhenius equation:
k = A * e^(-Ea/RT)
Where k is rate constant
A is frequency factor (1.5x10¹¹s⁻¹)
Ea is activation energy = 55800J/mol
R is gas constant (8.314J/molK)
And T is absolute temperature (24°C + 273 = 297K)
Replacing:
k = 1.5x10¹¹s⁻¹ * e^(-55800J/mol/8.314J/molK*297K)
k = 1.5x10¹¹s⁻¹ * 1.53x10⁻¹⁰
k = 23.0 s⁻¹ is rate constantWhat process transfers water from the atmosphere to the hydrosphere
A evaporation
B runoff
C precipitation
D currents
Answers
Why are bacteria are able to evolve rapidly
Answers
please name these chemicals.

Answers
Answer: Carbon,Hydrogen, Chlorine
Explanation:
C=Carbon
H=Hydrogen
Cl=Chlorine
Hope this helps :)
Calculate the approximate mass of ammonium chloride needed for 25.00 ml of a 0.1000 m solution by substituting the value of the molecular weight of ammonium chloride into the following equation:
Answers
Approximately 0.135 g of ammonium chloride is needed for 25.00 mL of a 0.1000 M solution.
To calculate the mass of ammonium chloride needed for 25.00 mL of a 0.1000 M solution, we first need to determine the number of moles of ammonium chloride required:
moles of ammonium chloride = volume of solution (in L) x concentration of solution (in mol/L)
Since the volume of solution is given in mL, we need to convert it to L:
25.00 mL = 0.02500 L
Now we can substitute the given concentration of the solution to get the number of moles of ammonium chloride:
moles of ammonium chloride = 0.02500 L x 0.1000 mol/L = 0.00250 mol
Finally, we can calculate the mass of ammonium chloride needed using its molecular weight (53.49 g/mol):
mass of ammonium chloride = moles of ammonium chloride x molecular weight of ammonium chloride
mass of ammonium chloride = 0.00250 mol x 53.49 g/mol = 0.135 g (to three significant figures)
Therefore, approximately 0.135 g of ammonium chloride is needed for 25.00 mL of a 0.1000 M solution.
learn more about ammonium here:
https://brainly.com/question/23387600
#SPJ11
the complete question is:
Calculate the approximate mass of ammonium chloride needed for 25.00 ml of a 0.1000 m solution by substituting the value of the molecular weight of ammonium chloride into the following equation:
mass = molarity x volume x molecular weight
Please show all your work and include the units in your answer.
………………………………………………………….
Answers
No idea what this is for, but I hope you have an amazing day:)
which compound will form the most intensely colored 0.01 m aqueous solution?
Answers
The compound that will form the most intensely colored 0.01 M aqueous solution will depend on the specific compound and its ability to exhibit color in solution.
The compound that will form the most intensely colored 0.01 M aqueous solution will depend on the specific compound and its ability to exhibit color in solution. Without knowing the specific compounds being considered, it is difficult to provide a definitive answer. Different compounds can exhibit a wide range of colors based on their molecular structure and electronic transitions.
In general, compounds with highly conjugated systems or transition metal complexes tend to exhibit intense colors in solution. These compounds often have extended delocalized π-electron systems that allow for absorption of light in the visible range, resulting in the observed color.
To determine which compound would form the most intensely colored solution, it would be necessary to consider the specific compounds and their molecular structures, including the presence of conjugated systems or transition metal ions. Conducting experiments or consulting references specific to the compounds in question would provide more accurate information regarding their coloration in aqueous solution.
To learn more about ions, click here: brainly.com/question/10942834
#SPJ11
the particle that move around the nucleus is are
Answers
Answer:
electrons
The nucleus contains two types of subatomic particles, protons and neutrons. The protons have a positive electrical charge and the neutrons have no electrical charge. A third type of subatomic particle, electrons, move around the nucleus. The electrons have a negative electrical charge
Explanation:
HOPE ITS HELPFUL FOR U IF YES PLZ MARK ME AS BRILLIANT
carbon dioxide has one more resonance form than ozone. explain why this structure is not possible for ozone.
Answers
Carbon dioxide has one more resonance form than ozone.
Define resonance.
In resonance, more than one or two Lewis structures can each represent a single substance (or molecule). The overall Lewis structure of a chemical (or molecule) is a mix of the two.
One of the three resonance structures in carbon dioxide, or CO2, is a significant contributor. Four valence electrons from carbon and six from each oxygen atom make up the total of 16 in the CO2 molecule. All three resonance structures have full octets of atoms, however structure 1 will be more stable and so contribute more because it lacks charge separation.
Because both oxygen atoms have formal charges, structures 2 and 3 exhibit charge separation. Furthermore, the stability of these two complexes is further diminished by oxygen having a positive charge.
We have two significant resonance structures for ozone, both of which are equally important to the molecule's overall hybrid structure. The required 18 valence electrons are provided by both configurations (6 from 3 bonds and 12 as lone pairs placed on the oxygen atoms).
To learn more about valence electrons use link below:
https://brainly.com/question/371590
#SPJ4

The price of gold (molar mass = 196.97 g/mol) has varied so much over the last 30 years that with $100 you could buy as much as 2.6 troy ounces (81 g) of gold or as little as 0.13 troy ounces (4.0 g). calculate the amount in moles that these two masses of gold represent.
Answers
Answer:
0.41 and 0.02 moles Au
Explanation:
Moles:
81 grams (2.6 troy oz):
mole Au = 81g/(196.97 g/mole) = 0.41 moles Au
4.0g (0.13 oz):
mole Au = 4.0g/(196.97 g/mole) = 0.020 moles Au
What is the valency of aluminum in aluminum chloride?
A. 1
B.2
C.3
D.4
Answers
Answer:
C.3
13AL
2.8.3
The number of electrons from the outermost shell is the valence of the element.
Week 8 of Quarter 2
Learning Task 1. From the picture given, write at least 5 observations in your
answer sheet.
pasagot po please.

Answers
Answer:
blurred an pic mo paki ayos
I have a 1cm3 cube shaped piece of gold (Au) at 900oC. The atomic weight Au is 196. 9 g/mol and its density at 900oC is 18. 63g/cm3.
a) If the formation energy for vacancies are 0. 98eV/atom, what is the number of vacancies in my piece of gold
Answers
The number of vacancies comes out to be 3.52 *10¹⁸/cm³ that can be shown in the below expalnation.
The number of vacancies can be calculated using the below formula-
Nv = N exp (-Qv / kT)
= (Na x D / A) exp (-Qv / kT)
It is given that,
D = 18.63 g/cm³
Na is known which is 6.022*10²³ atoms/mol also called Avogadro's number.
Qv = 0.98 eV/atom
T = 1173 K
K = 8.65*10⁻⁵ eV/atom-K
Substituting these values in the above equation as follows-
Nv = (6.02*10²³ atoms/mol) (18.63 g/cm3) / (1969.9 g/mol) exp (0.98 ev/atom / (86.2*10⁻⁵ ev atom -K)(1173 K)
= 3.52 *10¹⁸/cm³
To learn more about Avogadro's number check the link below-
https://brainly.com/question/1513182
#SPJ4
A 10.0 g sample of an unknown liquid is vaporized at 120.0°C and 5.0 atm. The volume of the vapour is found to be 568.0 mL. The liquid is determined to be made up of 84.2% carbon and 15.8% hydrogen. What is the molecular formula for the liquid?
Answers
Answer:
molecular formula of liquid = C₈H₁₈
Explanation:
First we determine the empirical formula of the liquid:
Number of moles of each element present in the liquid = % mass / molar mass
For Carbon, (molar mass = 12.01 g/mol) : 84.2/12.01 =7.011 moles
For Hydrogen (molar mass = 1.01 g/mol) : 15.8/1.01 = 15.643
Simplest mole ratio of the elements, C : H is given by:
C = 7.011/7.011 = 1.0
H = 15.643/7.011 = 2.23
Multiplying through with 5, C:H = 5:11
Therefore, empirical formula is C₅H₁₁
The molecular mass of the liquid is next determined:
Using PV = nRT to find the number of moles of the liquid present
P = 5.0 atm; V = 568.0 mL = 0.568 L; R = 0.082 L*atmmol⁻¹ K⁻¹; T = 273 + 120 = 393 K
n = PV/RT = (5*0.568)/0.082*393
n = 0.088 moles
Molar mass of liquid = mass/no of moles = 10.0 g/ 0.088 moles = 113.63 gmol⁻¹
Molecular formula = n(empirical formula)
Molar mass of empirical formula, C₅H₁₁ = 71 gmol⁻¹
n = molecular mass/empirical mass = 113.63/71 = 1.6
Therefore, molecular formula = 1.6*(C₅H₁₁) = C₈H₁₈
PLEASE HELP ASAP!!!!
ANSWERS FOR THE LAST PHOTO
W
X
Y
Z

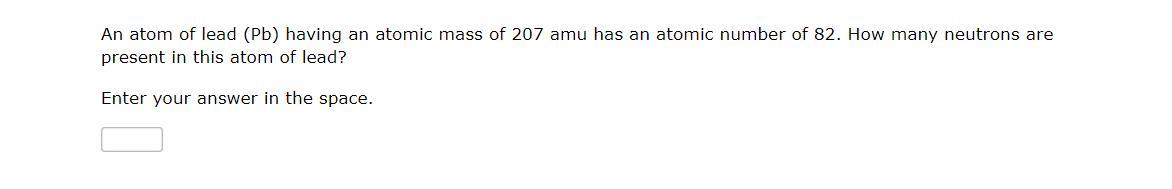



Answers
An atom is made up of three subatomic particles namely; protons, neutrons and electrons.
The proton is the positively charged particle in the nucleus of the atom while the electron is the negatively charged particle surrounding the nucleus.
The number of neutrons can be calculated by subtracting the atomic number of the element from the mass number.
In question 1 of the above image, no of neutrons = 39 - 19 = 20 neutronsIn question 2, no of neutrons = 207 - 82 = 125 neutronsLearn more about neutrons at: https://brainly.com/question/28992636
#SPJ1
Match each energy transformation to the correct image. ANSWER FAST PLS!!! THIS IS FOR 20 POINTS!!! AND I WILL MARK BRAINLIEST IF CORRECT!!! this is on edementum btw

Answers
The Light Is- Electric To Radiant
Car Battery- Chemical To Electric
Wind Thingy- Motion To Electric
Fan- Electric To Motion
Guitar- Electric To Sound
Hope This Helps!!
The atomic radius of atoms increases in groups of elements on the Periodic Table of the Elements. Which best describes what tends to occur in groups that results in an increase in atomic radius?
Answers
The attraction between the nucleus and electrons decreases tends to occur in groups that results in an increase in atomic radius.
Magnesium atoms have atomic radii that are lower than those of sodium atoms. The magnesium atom's smaller radius is mainly due to the fact that it has two more electrons in its outermost energy level than the sodium atom does.The attractive interactions between the nucleus and the additional electrons in the magnesium atom make them weaker, which leads to a reduced atomic radius. An element's atomic radius serves as a gauge for the size of its atoms.
The atomic radius of atoms increases in groups of elements on the Periodic Table of the Elements. Which best describes what tends to occur in groups that results in an increase in atomic radius?
Learn more about atomic radius here:
https://brainly.com/question/29440273
#SPJ4