What do all volcanos emit?
Answers
Answer:
By far the most abundant volcanic gas is water vapor, which is harmless. However, significant amounts of carbon dioxide, sulfur dioxide, hydrogen sulfide and hydrogen halides can also be emitted from volcanoes.
Related Questions
What property of matter does not change even though the appearance may change?
Answers
Answer:
The law of the conservation of mass states that matter is neither created nor destroyed, only converted to other forms. Therefore, the mass never changes, even if its appearance does.
Explanation:
How is a rainbow made
Answers
which of your body structures was the effector in the reaction time test? what was your motor response?
Answers
Skeletal muscle was the Effector in the reaction time test and motor response reflects the muscular component of reaction time.
The Effector in the reaction time test was the skeletal muscle in the finger which is used to press the button. muscle and glands produces a specific response to a stimuli's and the motor response was reaction time test is the time between electromyographic activity and movement and the motor response is the response which reflects the skeletal muscle component of reaction time.
learn more about Effector
brainly.com/question/3190796
#SPJ4
for the pair of compounds co2 or no2 the one with the highest boiling point is
Answers
NO2 has a higher boiling point than CO2
What do you notice about the straw in the glass of water? brainpop science

Answers
It appears that the straw in the glass of water is bent or refracted at the water-air interface. This is a result of the phenomenon known as refraction. Refraction occurs when light travels from one medium to another with a different optical density, causing the light rays to change direction.
Based on the provided image from BrainPOP Science, it appears that the straw in the glass of water is bent or refracted at the water-air interface. This is a result of the phenomenon known as refraction. Refraction occurs when light travels from one medium to another with a different optical density, causing the light rays to change direction.
In this case, as the light passes from the water to the air, it undergoes refraction because the speed of light is different in water compared to air. The change in speed causes the light rays to bend as they cross the water-air boundary. This bending of light is what makes the straw appear bent or displaced when viewed through the glass of water.
The phenomenon of refraction is a common optical effect and can be observed in various situations where light passes through different mediums with varying optical properties.
For more question on density
https://brainly.com/question/26364788
#SPJ8
how many seconds are there in 45 days?
Answers
Answer:3,888,000seconds
Explanation:
60secx60minsx24 hoursx45days=3,888,000
Williamson synthesis of 1-isopropoxy-1-methylcyclopentane. O Williamson ether synthesis would give a poor yield of product as the product does not have Markovnikov orientation.O Williamson ether synthesis would give a poor yield of product as the product does not have anti-Markovnikov orientation. O Williamson ether synthesis would give a poor yield of product as the halide is on a 3º carbon.O Williamson ether synthesis would give a poor yield of product as the halide is on a 2° carbon.
Answers
Williamson ether synthesis would give a poor yield of product as the halide is on a 2° carbon. is true about the synthesis of 1-isopropoxy-1-methylcyclopentane.
The Williamson ether synthesis is a method for the synthesis of ethers using an alcohol and an alkyl halide. The reaction proceeds through a nucleophilic substitution mechanism and the ether product is obtained with the alcohol and halide groups in the anti-Markovnikov orientation. The reactivity of the alkyl halides used in this reaction follows the order: primary > secondary >> tertiary. Therefore, the reaction of a secondary halide such as 2° carbon halide will give a poor yield of product as the reactivity of 2° carbon halide is less compared to primary halide. As a result, the reaction is less efficient and the yield of the product is lower.
To know more about Williamson ether synthesis click below:
https://brainly.com/question/29434473#
#SPJ4
an oil drum weights 11.8kg. when it is filled, octane it weighs 55.32kg. how many molecules of pentane c5h12 exist in this oil drum?
Answers
Question: an oil drum weights 11.8kg. when it is filled, pentane it weighs 55.32kg. how many molecules of pentane c5h12 exist in this oil drum?
Answer:
3.64×10²⁶ molecules
Explanation:
Applying,
n = M/M.M................... Equation 1
Where n = number of moles of pentane, M = mass of pentane, M.M = reacting mass of pentane
From the question,
Given: M = 55.32-11.8 = 43.52 kg = 43520 g
Constant: M.M of octane (C₅H₁₂) = [(12×5)+(1×12)] = 60+12 = 72 g/mol
Substitute these values into equation 1
n = 43520/72
n = 604.44 moles
Therefore,
number of molecules = n×(6.02×10²³)
number of molecules = 604.44(6.02×10²³)
number of molecules = 3.64×10²⁶ molecules
at what points does the object accelerate please explain
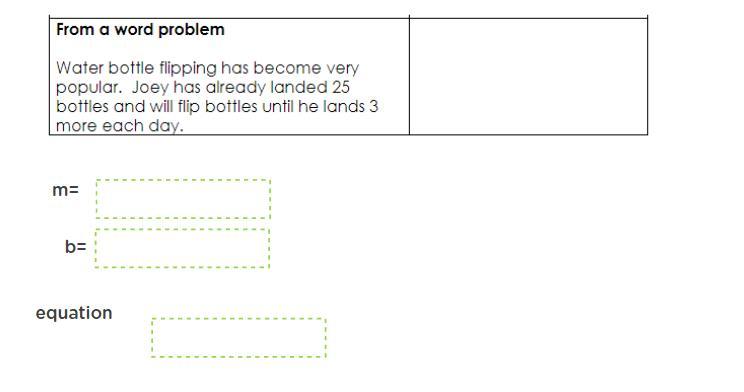
Answers
Answer:
M=3
B=25
Equation= 3x+25
Explanation:
M is your slope and if you follow the slope- intercept formula (y=m+b) you just need to plug in the numbers.
Thus your answer:
M=3
B=25
Equation= 3x+25
use the periodic table to select the element that best fits each of the following descriptions. which element below has properties of both metals and nonmetals?
zinc aluminum copper boron
Answers
Answer: Boron is the element which has properties of both metals and nonmetals.
Explanation:
Metals are defined as the elements which loose electrons to attain stable electronic configuration. They attain positive charge and form cation. Example: Zinc (Zn), Aluminium (Al) , copper (Cu)
Non-metals are defined as the elements which gain electrons to attain stable electronic configuration. They attain negative charge and form anion. Example: Chlorine (Cl) , Sulphur (S)
Metalloids are defined as the elements which show properties of both metals and non-metals. There are 7 metalloids in the periodic table. They are Boron (B) , Silicon (Si) , Germanium (Ge) , Arsenic (As) , Antimony (Sb), Tellurium (Te) and Polonium (Po).
Thus boron is the element which has properties of both metals and nonmetals.
Answer:
The answer is A boron!!!!!!
Explanation:
i took the quiz on ed
what would ebony do in water
Answers
Explanation:
It should be seen that perhaps the intensity of Ebony timber is greater than the concentration. This can drown if this is heavier than water, but that will floating whether it is not viscous than air.
Which atom absorbs more energy- one in which an electron moves from the the second shell to the third shell, or an atom which an electron moves from the first to the third shell?
Answers
An atom which an electron moves from the first to the third shell atom absorbs more energy.
An atoms may occupy different energy states . The energy states are discrete , that means they occur at specific values only. Therefore an atom can only move to a new energy level if it absorbs or emits an amount of energy that exactly corresponds to the difference between two energy levels.
The lowest possible energy levels that the atom can occupy is called ground state.The energy levels which is higher to the ground state is called excited state.
The more energy absorb when electron move from first to third because in this second energy level have to pass to reach at third energy level.
learn about atoms
https://brainly.com/question/1566330
#SPJ4
Q-3 Determine the fugacity in atm for pure ethane at 310 K and 20.4 atm and change in the chemical potential between this state and a second state od ethane where temperature is constant but pressure is 24 atm.
Answers
The fugacity in atm for pure ethane at 310 K and 20.4 atm is given by the equation: f = 20.4 exp (-Δg1/RT). The change in chemical potential between this state and a second state of ethane where the temperature is constant but the pressure is 24 atm is -0.0911RT.
Fugacity is a measure of the escaping tendency of a component in a mixture, which is defined as the pressure that the component would have if it obeyed ideal gas laws. It is used as a correction factor in the calculation of equilibrium constants and thermodynamic properties such as chemical potential. Here we need to determine the fugacity in atm for pure ethane at 310 K and 20.4 atm and the change in the chemical potential between this state and a second state of ethane where the temperature is constant but the pressure is 24 atm. So, using the formula of fugacity: f = P.exp(Δu/RT) Where P is the pressure of the system, R is the gas constant, T is the temperature of the system, Δu is the change in chemical potential of the system. Δu = RT ln (f / P)The chemical potential at the initial state can be calculated using the ideal gas equation as: PV = nRT
=> P
= nRT/V
=> 20.4 atm
= nRT/V
=> n/V
= 20.4/RT The chemical potential of the system at the initial state is:
Δu1 = RT ln (f/P)
= RT ln (f/20.4) Also, we know that for a pure substance,
Δu = Δg. So,
Δg1 = Δu1 The change in pressure is 24 atm – 20.4 atm
= 3.6 atm At the second state, the pressure is 24 atm.
Using the ideal gas equation, n/V = 24/RT The chemical potential of the system at the second state is: Δu2 = RT ln (f/24) = RT ln (f/24) The change in chemical potential is Δu2 – Δu1 The change in chemical potential is
Δu2 – Δu1 = RT ln (f/24) – RT ln (f/20.4)
= RT ln [(f/24)/(f/20.4)]
= RT ln (20.4/24)
= - 0.0911 RT Therefore, the fugacity in atm for pure ethane at 310 K and 20.4 atm is:
f = P.exp(Δu/RT)
=> f
= 20.4 exp (-Δu1/RT)
=> f
= 20.4 exp (-Δg1/RT) And, the change in the chemical potential between this state and a second state of ethane where the temperature is constant but pressure is 24 atm is -0.0911RT. Therefore, the fugacity in atm for pure ethane at 310 K and 20.4 atm is given by the equation: f = 20.4 exp (-Δg1/RT). The change in chemical potential between this state and a second state of ethane where the temperature is constant but the pressure is 24 atm is -0.0911RT.
To know more about chemical potential visit:-
https://brainly.com/question/31100203
#SPJ11
Convert 0.057 m to km
Answers
Answer:
5.7e-5
Explanation:
\(\frac{0.057}{1000} \\= 5.7\times 10^-5 km\)
0.057 meters is equivalent to 0.000057 kilometers.
The metric system is a decimal-based system of measurement where prefixes are used to indicate different magnitudes of the base unit. In the case of length or distance, the base unit is the meter (m), and the prefix "kilo-" represents a factor of 1000.
To convert a smaller unit, such as meters, to a larger unit, such as kilometers, we divide by the appropriate conversion factor, divide by 1000 since there are 1000 meters in 1 kilometer.
To convert meters (m) to kilometers (km), divide the value in meters by 1000 since there are 1000 meters in 1 kilometer.
Given that we want to convert 0.057 m to km, calculate it as follows:
0.057 m ÷ 1000 = 0.000057 km
To learn more about the kilometer, follow the link:
https://brainly.com/question/13987481
#SPJ6
define the term "filtration"
Answers
Answer:
the action or process of filtering something. hope this helps :D
hey guys i need help with this story problem
A student measured a cylinder to have a length of 4.21 cm and a diameter of 1.29 cm. What is the volume of the cylinder? Include units (cm3) and round to the proper number of significant digits. V = π x r^2 x L
Answers
A student measured a cylinder to have a length of 4.21 cm and a diameter of 1.29 cm. The volume of the cylinder is \(V \approx 5.5 \mathrm{~cm}^3\).
\(V=\pi\left(\frac{d}{2}\right)^2 h=\pi \cdot\left(\frac{1.29}{2}\right)^2 \cdot 4.21 \approx 5.50239 \mathrm{~cm}^3\)
A cylinder's volume refers to the amount of interior room it has to hold a given quantity of material. To put it another way, a cylinder's volume is how much it can hold. You can store any one of the three forms of matter—solid, liquid, or gas—within the confines of a cylinder. You cannot hold any liquid, solid, or gas in a two-dimensional cylinder, hence this capacity can only be observed in a three-dimensional cylinder.
Two congruent and parallel identical bases make up a complete three-dimensional cylinder. The right circular cylinder is what is meant by this. Each line segment makes up the lateral curved surface, which is perpendicular to the bases, of a right circular cylinder, which has circular bases. The proper circular cylinders might have crossed your path on a regular basis. Can shapes, paper roll shapes, straight glass, and many other things.
Learn more about volume of the cylinder https://brainly.com/question/16134180
#SPJ9
if you completely react 6 moles of h2 gas (with n2 as excess), how many moles of nh3 can be produced?
Answers
The number of moles of NH3 can be produced are 4 moles.
What is a mole?
The mole is the amount of substance in a system that includes the same number of elementary entities as there are atoms in 0.012 kilogram of carbon 12; it is denoted by the sign "mol."
What is a limiting factor?
Limiting factors are reactants or reagents that are used by a chemical reaction before other reactants. The limiting factor is the reactant or reagent with the lowest supply in terms of its required ratio in comparison to other reactants in the system.
According to the question;
Balanced equation would be :
\(3H_2 + N_2\) ⇒ \(2NH_3\)
Here, \(H_2\) is a limiting factor
No. of moles of ammonia formed when 3 moles of \(H_2\) react with excess of \(N_2\) =2
applying unitary method:
No. of moles of ammonia formed when 6 moles of \(H_2\) react with excess of \(N_2\)= \(\frac{2}{3 } * 6\) = 4moles.
The number of moles of NH3 can be produced are 4 moles.
To know more about limiting factor, check out:
https://brainly.com/question/14222359
#SPJ4
a gas occupying 75 ml at standard conditions is heated to 17°c while the pressure is reduced to 0.97 atm. what is the new volume occupied by the gas
Answers
Under ordinary circumstances, a gas that takes up 75 ml is heated to 17 °C and the pressure is lowered to 0.97 atm. The gas has now filled a capacity of 79.9 ml.
To solve this problem, we can use the combined gas law, which relates the pressure, volume, and temperature of a gas:
(P₁ × V₁) / T₁ = (P₂ × V₂) / T₂
where P1, V1, and T1 are the initial pressure, volume, and temperature, respectively, and P₂, V₂, and T₂ are the final pressure, volume, and temperature, respectively.
We are given P₁ = 1 atm (standard conditions), V₁ = 75 ml, T1 = 273 K (standard conditions + 0°C), P2 = 0.97 atm, and T₂ = 17°C + 273 = 290 K.
Substituting these values into the formula and solving for V₂, we get:
(1 atm × 75 ml) / 273 K = (0.97 atm × V₂) / 290 K
Simplifying and solving for V2, we get:
V₂ = (1 atm × 75 ml × 290 K) / (273 K × 0.97 atm) = 79.9 ml
Therefore, the new volume occupied by the gas is approximately 79.9 ml (three significant figures).
Learn more about pressure here:
https://brainly.com/question/30673967
#SPJ4
compounds y and z both have the formula c9h18. both y and z react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methyloctane. the heat of hydrogenation of y is less than that of z. y and z each undergo hydroboration/oxidation to give a primary alcohol (oh attached to a primary carbon). what is the structure of y?
Answers
Both molecules can be hydrogenated to produce the same result, and hydroboration and oxidation both produce primary alcohols that are geometric isomers, of which Y is the trans isomer.
Explain about the hydrogenation?Molecular hydrogen (H2) and another substance or element undergo a chemical reaction known as hydrogenation, typically in the presence of a catalyst such as nickel, palladium, or platinum. The method is frequently used to saturate or decrease organic molecules.
To solidify a liquid oil into a fat. When solid fats with the right consistency are expensive or unavailable, hydrogenation, sometimes in combination with other processes like interesterification or fractionation, may provide a method to synthesize the desired fat.
The FA chains linked to the TAG backbone undergo hydrogenation, a chemical reaction that adds hydrogen to their unsaturated bonds. An unsaturated fat can become a saturated fat in this manner, raising its melting point.
To learn more about hydrogenation refer to:
https://brainly.com/question/12833896
#SPJ1
Hydrogen bonding is strongest between molecules of
A) H2S
B) H2Se
C) H2O
D) H2Te
Answers
I hope this helped out, have a nice day! <3

formic acid is partly responsible for the sting of ant bites. by mass, formic acid is 26.10%c , 4.38%h , and 69.52%o . the molar mass of formic acid is 46.02 g/mol . read section 5.3. you can click on the review link to access the section in your etext. part a find the molecular formula of formic acid. express your answer as a chemical formula. enter the elements in the order c, h, and o.
Answers
Part a: Find the molecular formula of formic acid. Express your answer as a chemical formula. Enter the elements in the order C, H, and O.The molecular formula of formic acid is HCOOH.How to calculate the molecular formula of formic acid.
Percent composition of formic acid: C = 26.10%, H = 4.38%, and O = 69.52%.The molar mass of formic acid is 46.02 g/mol.The molar mass of a compound is the sum of the atomic masses of all the atoms in a molecule.Using these values, we can calculate the empirical formula and molecular formula of formic acid.1. Calculate the empirical formula.
The empirical formula is the simplest whole-number ratio of atoms in a compound. To calculate the empirical formula, we assume 100 grams of the compound and calculate the number of moles of each element present. Then we divide each mole value by the smallest value to get a simple ratio.C = 26.10 gH = 4.38 gO = 69.52 gWe can convert these values to moles by dividing by the atomic masses:C: 26.10 g / 12.01 g/mol = 2.17 molH: 4.38 g / 1.01 g/mol = 4.34 molO: 69.52 g / 16.00 g/mol = 4.35 mol.
Next, we divide each of these mole values by the smallest value, which is 2.17:Empirical formula = HCOOH.2. Calculate the molecular formulaThe molecular formula is a multiple of the empirical formula. To calculate the molecular formula, we need to know the molar mass of the compound.Molar mass of empirical formula = 12.01 + 1.01 + 16.00 + 16.00 + 1.01 = 46.03 g/molThe molecular formula is the empirical formula multiplied by a whole number that gives the molar mass of the compound. We can calculate this factor by dividing the molar mass of the compound by the molar mass of the empirical formula:Factor = 46.02 g/mol / 46.03 g/mol = 0.9998, which is approximately 1.The molecular formula is HCOOH.
Learn more about formic acid:
https://brainly.com/question/24987685
#SPJ11
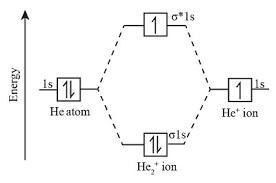
construct the molecular orbital diagram for he+2 .
Answers
The molecular orbital diagram of the specie that is shown in the image attached.
What is molecular orbital?A molecular orbital (MO) is a location in a molecule where there is a high likelihood of encountering electrons. A development of atomic orbital theory, molecular orbital theory analyzes the behavior of electrons in molecules by taking into account how atomic orbitals overlap to generate molecular orbitals.
According to the molecular orbital theory, molecular orbitals that cover the complete molecule are created by combining the atomic orbitals from various atoms. Bonding and antibonding molecular orbitals are created by the conjunction of atomic orbitals.
Learn more about molecular orbital:https://brainly.com/question/29642622
#SPJ4
Which element is not abbreviated based on an ancient name?.
Answers
Radium (Ra) is an example of a chemical element which is not abbreviated based on an ancient name.
What is a chemical element?A chemical element can be defined as a pure substance that comprise atoms with the same atomic number (number of protons) in its atomic nucleus.
The examples of a chemical element.
In Chemistry, some examples of a chemical element include the following:
Carbon (C)Iron (Fe)Sodium (Na)Radium (Ra)Generally, a chemical symbol is typically used in chemistry to abbreviate an atom of a given element or a chemical element. Also, some chemical elements are not abbreviated based on an ancient name such as Radium (Ra).
Read more on chemical elements here: https://brainly.com/question/377844
What is the mole fraction of C12H22011?
Answers
The formula is
\(\boxed{\sf \chi_A=\dfrac{\chi_A}{\chi_A+\chi_B}}\)
Denominator will be same for all
Find that
moles of solution=12+22+11=45Now
#C
\(\\ \rm\Rrightarrow \chi_C=\dfrac{12}{45}\)
#H
\(\\ \rm\Rrightarrow \chi_H=\dfrac{22}{45}\)
#O
\(\\ \rm\Rrightarrow \chi_O=\dfrac{11}{45}\)
which pollutant would you suggest the government try to limit in order to slow down the breakdown of the marble on the taj mahal
Answers
Answer:
SO2
Explanation:
SO2 causes acid rain which is leading to dissolving of the marble on the Taj Mahal
SO2 reacts with water to come down as sulfuric acid during acid rains
If the size of an object decreases, volume does what?
Answers
Answer:
I'm pretty sure the volume decreases because the volume is like how much space it takes up. Sorry if im wrong.
The reaction between C₂H2O, and O₂ is represented by the balanced equation above. In an experiment, 0.30 mol of CO₂ was produced from the reaction of 0.05 mol of C₂H₂O with excess
O₂. The reaction was repeated at the same temperature and in the same container, but this time 0.60 mol of CO₂ was produced. Which of the following must be true?
Answers
There must have been 0.10mol of \(C_{2} H_{2} O\) in the container at the beginning.
\(C_{2} H_{2} O\) + \(2O_{2}\) = \(2CO_{2}\) + \(H_{2} O\)
The above reaction makes it quite evident that 1 mol of \(C_{2} H_{2} O\) combines to create 2 mol of \(CO_{2}\) and \(O_{2}\) is given in excess that \(C_{2} H_{2} O\) alone controls a product's formation. Therefore, here, O is an excess reactant and \(C_{2} H_{2} O\) is a limiting reactant.
It takes 6 times as much \(C_{2} H_{2} O\) to produce 1 mol of \(CO_{2}\) from 0.05 mol of \(C_{2} H_{2} O\).
Now, 0.6 divided by 6 mol of reactant is required for 0.60 moles of \(CO_{2}\)to produce, which translates to
moles of \(C_{2} H_{2} O\) = 0.6/6 = 0.1 mol .
Therefore, \(C_{2} H_{2} O\) must have been present in the container in an initial concentration of 0.10mol.
Learn more about mole calculations here-
https://brainly.com/question/1578931
#SPJ9
Fuel-efficient cars help decrease the global dependency on nonrenewable resources because they use less _______ than other vehicles to travel the same distance.
Answers
Fuel-efficient cars help decrease the global dependency on nonrenewable resources because they use less fuel than other vehicles to travel the same distance.
What are non renewable resources?Nonrenewable resources are natural resources that cannot be readily replaced or regenerated, such as fossil fuels (coal, oil, and natural gas), minerals, and some metals. These resources are finite and once they are used they cannot be replenished. They are formed over millions of years and are essential for the functioning of modern society, but their extraction and use can have negative impacts on the environment.
To know more about fossil fuels, visit:
https://brainly.com/question/3371055
#SPJ1
Calculate: A. Mercury has a specific Heat Capacity of 0.14 J/goC. How much heat is needed to raise the thermometer temperature 15 oC to 95 oC. There is 5 g of mercury in the thermometer.
Answers
Answer:
\(\boxed {\boxed {\sf 56 \ Joules}}\)
Explanation:
We are given the mass, specific heat, and temperature, so we must use this formula for heat energy.
\(q=mc \Delta T\)
The mass is 5 grams, the specific heat capacity is 0.14 Joules per gram degree Celsius. Let's find the change in temperature.
ΔT= final temperature - initial temperature ΔT= 95°C - 15°C = 80°CWe know the variables and can substitute them into the formula.
\(m= 5 \ g \\c= 0.14 \ J/ g \ \textdegree C \\\Delta T= 80 \ \textdegree C\)
\(q= (5 \ g )( 0.14 \ J/ g \ \textdegree C ) ( 80 \ \textdegree C)\)
Multiply the first numbers. The grams will cancel.
\(q= 0.7 \ J/ \textdegree C(80 \ \textdegree C )\)
Multiply again. This time the degrees Celsius cancel.
\(q= 56 \ J\)
56 Joules of heat are needed.
Look at the reaction below. upper h subscript 2 upper s upper o subscript 4 (a q) plus upper c a (upper o upper h) subscript 2 (a q) right arrow upper c a upper s upper o subscript 4 (a q) plus 2 upper h subscript 2 upper o (l). which substance is the base in the reaction? 2h2o (l) h2so4 (aq) caso4 (aq) ca(oh)2 (aq)
Answers
In the given reaction, the substance that is the base of the reaction is \(CaSO_4\). The correct option is C.
What is a base?The base is a slippery liquid that is bitter and turns red litmus paper to blue.
Bases react with acids to form salts.
Then bases have pH above 7.
Examples of bases are calcium carbonate, sodium hydroxide, etc.
Thus, the correct option is C.
Learn more about bases
https://brainly.com/question/485375
Answer:
D. Ca(OH)2 (aq)
Explanation:
Correct on Edge 2022!!!
Good luck everyone, you got this! Have a great day!