Answers
In Schrodinger's model, instead of the atom only having electrons as particles that would be placed in certain orbits of fixed energy around the positive charged nucleus, he discovered that electrons actually will behave as standing waves that will be more likely, with a higher probability, to be found in some regiouns of space, which are called orbitals.
Related Questions
what would go in the red square?

Answers
0.86 moles of \(N_2\) and 1.72 moles of Li will react.
Calculation-We must place a 3 coefficient in front of Li in order to bring the equation into balance:
\(N_2 + 2Li = 2Li_3N\)
The balanced equation demonstrates that 2 moles of Li and 1 mole of N2 react to create 2 moles of Li3N. Therefore, we can apply the following dimensional analysis to determine how many moles of Li will react with 0.86 moles of N2:
\(0.86 mol N_2 x (2 mol Li / 1 mol N_2) = 1.72 mol Li\)
What is an equation, in your opinion?A declaration that two expressions with variables or integers are equal. In essence, equations are questions and attempts to systematically identify the solutions to these questions have been the driving forces behind the creation of mathematics.
to know more about equations here:
brainly.com/question/29657983
#SPJ1
How many significant figures are in the following number? 3,000.0 x 10⁴ grams
Answers
Answer:
5 significant figures.
Explanation:
The decimal place makes the zeros significant.
When a solution is diluted with water, the ratio of the initial to final
volumes of solution is equal to the ratio of final to initial molarities
Select one:
True
-
Answers
The correct answer for this problem would be TRUE.
Explanation: it is true that when a solution is diluted with water, the ratio of the initial to final volumes of solution is equal to the ratio of final to initial molarities.
When a solution is diluted with water, the ratio of the initial to final volumes of solution is equal to the ratio of final to initial molarities. The statement is True.
Concentration refers to the amount of a substance in a defined space. Another definition is that concentration is the ratio of solute in a solution to either solvent or total solution.
There are various methods of expressing the concentration of a solution.
Concentrations are usually expressed in terms of molarity, defined as the number of moles of solute in 1 L of solution.
Solutions of known concentration can be prepared either by dissolving a known mass of solute in a solvent and diluting to a desired final volume or by diluting the appropriate volume of a more concentrated solution (a stock solution) to the desired final volume.
Learn more about Concentrations, here:
https://brainly.com/question/10725862
#SPJ3
How many water molecules are in 4 moles of water
Answers
Answer:
12.
Explanation:
There are 12 atoms within 4 water molecules.
Two clear solutions are placed in separate beakers. The first solution has a pH = 4, and the pH of the second solution is unknown.
If the two solutions are mixed and the resulting solution has a pH = 7, describe the second solution.
A) The second solution has a higher pH level than the first solution. It has a
pH that is weaker but still neutralized the first solution. It also has a higher
concentration of hydronium ions compared to the first solution.
B) The pH of the second solution is higher than the pH of the first solution. It
is also acidic and, therefore, has a higher concentration of hydroxide ions
compared to the first solution.
C) The second solution has a basic pH level. It was strong enough to
neutralize the first solution. It also has a higher concentration of hydroxide
ions compared to the first solution.
D) The second solution has and equivalent number of hydroxide and
hydronium ions. It has a pH level that is higher (stronger) than the first
solution.
Answers
Answer:
C.
Explanation:
When the pH is 7 there is equal amount of H+ and OH so the solution added must be strong enough to nuetralize the acid. So option A is out B is wrong because adding a acidic solution to an acidic solution wont nuetralize it, D is wrong because if the 2 solution was already equal in both it would essentaly be water. ALthough water would raise the pH it would not nuetralize it to a even 7.
Identify reactions types and balancing equations

Answers
Balance the following chemical equations:
1. N2 + 3 H2 → 2 NH3
Ex: Synthesis reaction
2. 2 KClO3 → 2 KCl + 3 O2
Single Replacement reaction
3. 2 NaF + ZnCl2 → ZnF2 + 2 NaCl
Decomposition reaction
4. 2 AlBr3 + 3 Ca(OH)2 → Al2(OH)6 + 6 CaBr2
Double Replacement reaction
5. 2 H2 + O2 → 2 H2O
Combustion reaction
6. 2 AgNO3 + MgCl2 → 2 AgCl + Mg(NO3)2
Synthesis reaction
7. 2 Al + 6 HCl → 2 AlCl3 + 3 H2
Decomposition reaction
8. C3H8 + 5 O2 → 3 CO2 + 4 H2O
Combustion reaction
9. 2 FeCl3 + 6 NaOH → Fe2O3 + 6 NaCl + 3 H2O
Double Replacement reaction
10. 4 P + 5 O2 → 2 P2O5
Synthesis reaction
11. 2 Na + 2 H2O → 2 NaOH + H2
Single Replacement reaction
12. 2 Ag2O → 4 Ag + O2
Decomposition reaction
13. C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Combustion reaction
14. 2 KBr + MgCl2 → 2 KCl + MgBr2
Double Replacement reaction
15. 2 HNO3 + Ba(OH)2 → Ba(NO3)2 + 2 H2O
Double Replacement reaction
16. C5H12 + 8 O2 → 5 CO2 + 6 H2O
Combustion reaction
17. 4 Al + 3 O2 → 2 Al2O3
Synthesis reaction
18. Fe2O3 + 2 Al → 2 Fe + Al2O3
Single Replacement reaction
Learn more about Chemical reactions, here:
https://brainly.com/question/29762834
#SPJ1
What electrically neutral atom has 30 neutrons and 25 electrons?
Answers
Answer:
Manganese (Mn)
Explanation:
We know it's manganese because we are told it is an electrically neutral atom. This means it has the same number of protons and electrons. If it has 25 electrons, it has 25 protons. Protons tell us the atomic number of the atom, which also tells us the name of the element. Manganese is element 25 on the periodic table.
What are the 2 consequences of global warming?
Answers
Answer:
Melting ice and rising seas
Explanation:
At the same time global warming causes polar ice sheets and glaciers to melt. The combination of these changes is causing sea levels to rise, resulting in flooding and erosion of coastal and low lying areas.
Momentum is calculated by multiplying
mass and speed
weight and speed
volume and velocity
mass and velocity
Answers
Answer
momentum is calculated using mass and velocity
The gas phase reaction of H2 with CO2 To produce H2O and CO has…
(Refer to the image, please)

Answers
The given reaction has ΔG value -12207KJ. Therefore, the given reaction is a spontaneous reaction as value of ΔG is negative.
A spontaneous process refers to anything that happens by itself, without external energy input. A ball is going to roll down an incline, water will flow downhill, ice will melt into water, radioactive elements will decay, and iron will rust, for instance. It is impossible for a reaction to not be spontaneous if it is exothermic (H negative) and increases the entropy for the system (S positive). The system's overall heat capacity is measured in enthalpy. The system's unpredictability is gauged by entropy.
ΔG=ΔH-T×ΔS
ΔG=11-298×41
= -12207KJ
Since ΔG is negative, reaction is spontaneous
To know more about spontaneous reaction, here:
https://brainly.com/question/31199175
#SPJ1
How many atoms of hydrogen would need to bond with a single atom of selenium to form a molecular compound?
Answers
Answer:
Hydrogen sulfide reacts with aqueous selenous acid to produce selenium disulfide:
H2SeO3 + 2 H2S → SeS2 + 3 H2O
Selenium disulfide consists of 8-membered rings. It has an approximate composition of SeS2, with individual rings varying in composition, such as Se4S4 and Se2S6. Selenium disulfide has been used in shampoo as an antidandruff agent, an inhibitor in polymer chemistry, a glass dye, and a reducing agent in fireworks.[15]
Selenium trioxide may be synthesized by dehydrating selenic acid, H2SeO4, which is itself produced by the oxidation of selenium dioxide with hydrogen peroxide:[17]
SeO2 + H2O2 → H2SeO4
Hot, concentrated selenic acid can react with gold to form gold(III) selenate.[18]
What is the formula of the compound in which the atom combining ratios are:
chlorine : oxygen : fluorine = 1:3:1
Enter elements in the order given:
Please help me with this
Answers
Answer:
Explanation:
The formula of the compound in which the atom combining ratios are:
chlorine : oxygen : fluorine = 1:3:1 is ClO3F i.e. perchloryl fluoride.
What is Chemical formula?The chemical formula is define as a method of providing information about chemical properties of atoms that make a particular chemical compound or molecule by chemical name and symbols.
There are mainly three types of chemical formula
Empirical formula - It can be defined as a simple representation of a relative number of each type of atom or ratio of the element in the compound.Molecular formula - It is defined as an indicator of simple number of all types of atom in a molecule of a molecular substance. Condensed formula - It is defined to characterize all types and spatial arrangement of bond in a simple chemical substance.Thus, the formula of the compound in which the atom combining ratios are:
chlorine : oxygen : fluorine = 1:3:1 is ClO3F i.e. perchloryl fluoride.
To learn more about chemical formula, refer to the link below:
https://brainly.com/question/11995171
#SPJ2
Arrange the following ions in order of increasing ionic radius: nitride ion, magnesium ion, fluoride ion, aluminum ion.
Answers
Explanation:
Nitride ion > Fluoride ion > Magnesium ion > Aluminum ion
b. Compare the hardness of the alkali metals family with the hardness of the alkaline earth metals family. (2 points)
Answers
Answer
Alkaline earth metals are more dense and harder than alkali metals
Explanation
The alkaline earth metals are the elements that belong to group 2 of the periodic table. This group of elements includes beryllium, magnesium, calcium, strontium, barium, and radium. Alkali metals, on the other hand, belong to group 1 of the periodic table. Alkaline earth metals have more density and are harder than alkali metals.
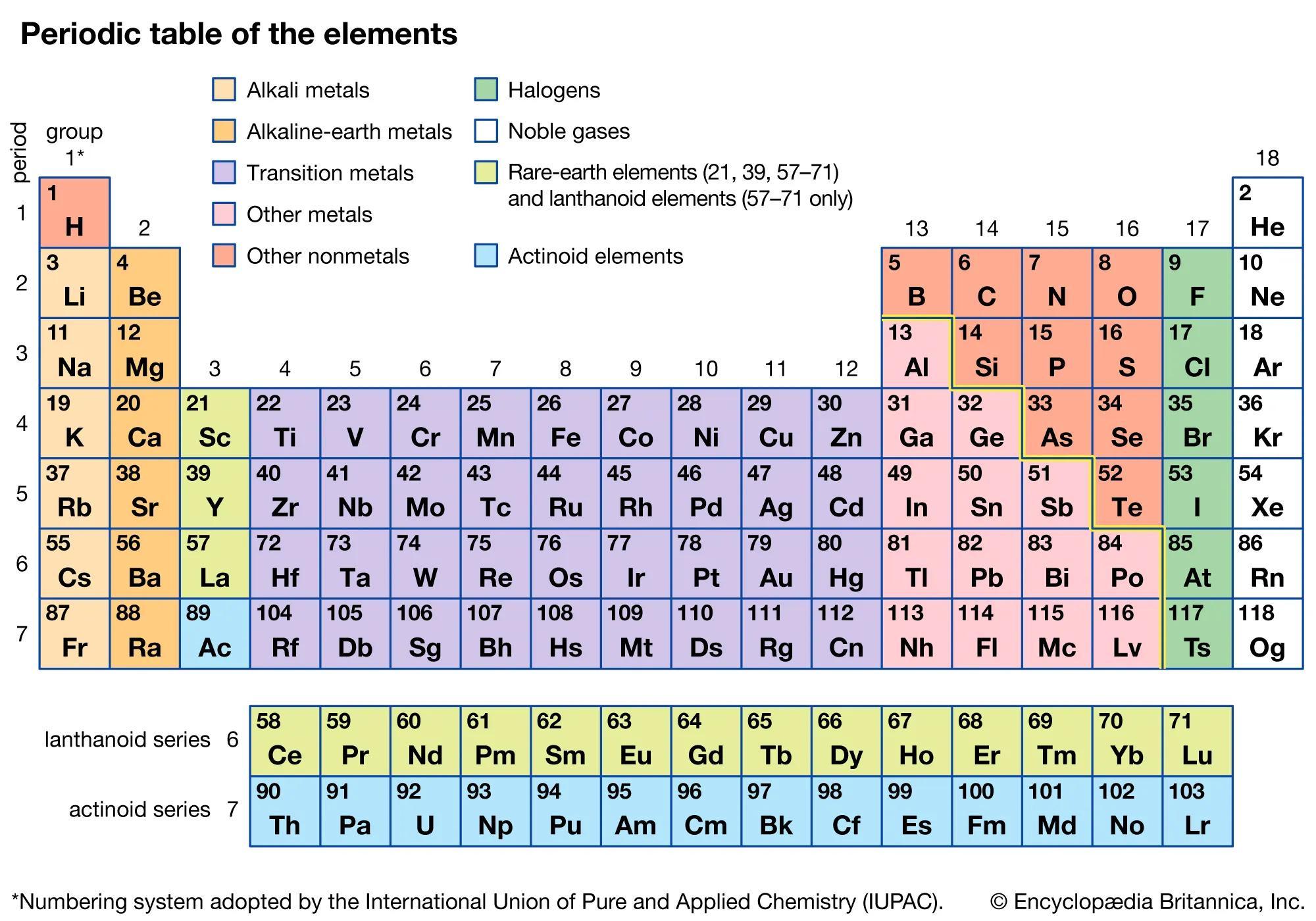
write the atomicity of oxygen
Answers
We made a bowl of jelly. What sort of change is this?
Answers
Answer:
Explanation:
It changes from a liquid to a solid. This change occurred due to the change in temperature. As the mixture cooled, the molecules condensed and the mixture formed a solid shape.Which formed the jelly.
Gasoline contains chemical potential energy. When you place gasoline in a car, you use up the gasoline and have to refill your gas tank. How can a teacher state that energy can neither be created nor destroyed in light of this observation? A) The teacher is only talking about chemical reactions. B) The teacher is stating a theory and not an actual law. C) This is an exception to the law of conservation of energy. D) The energy must be converted into different forms like motion and heat
Answers
Answer:
D
Explanation:
just took the test
PLS help I will give 100 points

Answers
Answer:
3700000000
Explanation: Move decimal to the right of the amount the exponent is for 10 Give brainliest pls
HELP ME OUT PLEASE!!!!!!!
This circuit shows a battery and wires connected to a lightbulb. The chemical energy in the battery is converted to:
A) radiant energy then electric energy
B) electric energy then chemical energy
C) electrical energy then radiant energy
D) radiant energy then mechanical energy

Answers
Answer:
electric energy then chemical energy
Explanation:
A battery is a device that converts chemical energy
PLEASE HELP QUICKK
Calculate the energy of combustion for one mole of butane if burning a 0.367 g sample of butane (C4H10) has increased the temperature of a bomb calorimeter by 7.73 °C. The heat capacity of the bomb calorimeter is 2.36 kJ/ °C.
Answers
The energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
To calculate the energy of combustion for one mole of butane (C4H10), we need to use the information provided and apply the principle of calorimetry.
First, we need to convert the mass of the butane sample from grams to moles. The molar mass of butane (C4H10) can be calculated as follows:
C: 12.01 g/mol
H: 1.01 g/mol
Molar mass of C4H10 = (12.01 * 4) + (1.01 * 10) = 58.12 g/mol
Next, we calculate the moles of butane in the sample:
moles of butane = mass of butane sample / molar mass of butane
moles of butane = 0.367 g / 58.12 g/mol ≈ 0.00631 mol
Now, we can calculate the heat released by the combustion of the butane sample using the equation:
q = C * ΔT
where q is the heat released, C is the heat capacity of the calorimeter, and ΔT is the change in temperature.
Given that the heat capacity of the bomb calorimeter is 2.36 kJ/°C and the change in temperature is 7.73 °C, we can substitute these values into the equation:
q = (2.36 kJ/°C) * 7.73 °C = 18.2078 kJ
Since the heat released by the combustion of the butane sample is equal to the heat absorbed by the calorimeter, we can equate this value to the energy of combustion for one mole of butane.
Energy of combustion for one mole of butane = q / moles of butane
Energy of combustion for one mole of butane = 18.2078 kJ / 0.00631 mol ≈ 2888.81 kJ/mol
Therefore, the energy of combustion for one mole of butane is approximately 2888.81 kJ/mol.
In conclusion, by applying the principles of calorimetry and using the given data, we have calculated the energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
For more questions on molar mass, click on:
https://brainly.com/question/837939
#SPJ8
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Why doesn't oil dissolve well in water?A.Both oil and water are nonpolar.B.Oil is nonpolar, but water is polar.C.Oil is polar, but water is nonpolar.D.Both oil and water are polar.
Answers
Answer
B. Oil is nonpolar, but water is polar.
Explanation
Water is held together by hydrogen bonds. Hence water is a polar molecule. Oils and fats do not have any polar part and so for them to dissolve in water they would have to break some of water hydrogen bonds.
So, non-polar molecules (e.g oil) can only mix well with other non-polar molecules and not with polar molecules like water. Therefore, this explains why oil doesn't mix well with water.
The correct answer is: B. Oil is nonpolar, but water is polar.
a weather balloon is inflated to a volume 2.2 10square3 L with 374g of helium. what is the density of helium in grams per liter
Answers
Answer:
Density = 0.17 g/L
Explanation:
It is given that,
Volume of the inflated balloon filled with Helium, \(V=2.2\times 10^3\ L\)
Mass, m = 374 g
We need to find the density of helium. It is equal to its mass per unit volume. It can be given by :
d =m/V
\(d=\dfrac{374\ g}{2.2\times 10^3\ L}\\\\=0.17\ g/L\)
So, the density of helium in the balloon is 0.17 g/L.
Consider how sodium chloride and butane interact with water: ______is more Iikely to dissolve In water because it is_____and is attracted to the______of water:
Answers
Sodium chloride is more likely to dissolve in water because it is polar and is attracted to the polar molecules of water. On the other hand, butane is nonpolar and is not attracted to the polar molecules of water, so it is less likely to dissolve.
Sodium chloride, also known as table salt, has ionic bonds, meaning it is composed of positive and negative ions. The positive sodium ion and negative chloride ion are attracted to the polar water molecules, which have a positive charge at one end and a negative charge at the other. This attraction between the ions of salt and the polar water molecules leads to the dissolution of salt in water.
Butane, on the other hand, is a nonpolar substance. It is composed of nonpolar carbon and hydrogen atoms arranged in a linear structure. These nonpolar atoms are not attracted to the polar water molecules, so they do not dissolve well in water. In fact, butane is a hydrocarbon and is one of the components of natural gas. It is a fuel that is used for heating and cooking, and it is not soluble in water.
In conclusion, the solubility of a substance in water depends on its molecular structure and the type of bonds it contains. Polar substances tend to dissolve well in water because they are attracted to the polar water molecules, while nonpolar substances are less likely to dissolve because they are not attracted to the polar water molecules.
To know more about solubility, visit:https://brainly.com/question/28170449
A 0.470 g sample of a metal, M, reacts completely with sulfuric acid according to M(s)+H2SO4(aq)⟶MSO4(aq)+H2(g) A volume of 217 mL of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. Atmospheric pressure is 756.0 Torr, and the temperature is 25 °C. The vapor pressure of water at 25 °C is 23.8 Torr. Calculate the molar mass of the metal.
Answers
The molar mass when rected with sulphuric acid to liberate 249 mL of hydrogen gas is 48 g/mol
The volume (V) = 249 mL = 249 / 1000 = 0.249 L
Pressure (P) = 1.0079 – 0.03167 = 0.97623 bar
The temperature (T) = 25.0 °C = 25 + 273 = 298 K
Gas constant (R) = 8.314×10¯² bar.L/Kmol
Number of mole (n) =?
n = PV / RT
n = (0.97623 × 0.249) / (8.314×10¯² × 298)
n = 0.0098 mole
How to determine the mole of the metal
Balanced equation
M(s) + H₂SO₄(aq) —> MSO₄(aq) + H₂(g)
From the balanced equation above,
1 mole of M reacts to produce 1 mole of H₂.
Therefore,
Mole of metal = 0.0098 mole
Mass of metal = 0.539 g
Molar mass is given as mass upon mole
Molar mass of metal = 0.470 / 0.0098
Molar mass of metal = 48 g/mol
Learn more about stoichiometry at:
brainly.com/question/14735801
#SPJ9
a student has a 5.00 gram sample of calcium chloride (CaCl2) solid. how many miles of calcium chloride are contained in this sample
Answers
Answer:
0.0451 moles ≅ 0.04 moles
Explanation:
We can calculate how many moles of calcium chloride (CaCl₂) are in 5.00 g sample, by dividing the mass into the molar mass (MM) of the compound:
moles = mass/MM
First, we calculate MM of CaCl₂ from the chemical formula, using the molar mass of the elements Ca and Cl:
MM CaCl₂ = (1 x MMCa) + (2 x MMCl) = 40 g/mol + ( 2 x 35.453 g/mol)
= 110.9 g/mol
Finally, we calculate the moles from the mass and MM:
moles of CaCl₂ = mass/(MM CaCl₂) = 5.00 g/(110.9 g/mol) = 0.0451 moles ≅ 0.04 moles
how many moles of water are there in :100g of H20 (b)1.00×10 rest to power 24 molecules of H20
Answers
Answer:
amole=5.55
b.mole=1.66
Explanation:
a.m=100g,M(H2O)=2+16=18
mole=100÷18=5.55
A sample of a certain lead compound contains 12.92 g of lead for 2 g of oxygen. A second sample has mass of 34.27 g and contains 14.39 g of oxygen. Are the two compound the same
Answers
The two chemical compounds are not the same, because their ratio is not equal. In both samples the composition of lead and oxygen is different.
What is a chemical compound?A chemical compound is a substance made of numerous similar molecules (or molecular entities) joined by chemical bonds and comprising atoms from various chemical elements. Therefore, a molecule made up of only one type of atom is not a compound. Chemical reactions, which may entail interactions with other molecules, can change a compound into a distinct substance. Atomic bonds may be broken or new ones created during this process.
What are the calculations?sample 1 = mass of lead / mass of oxygen = 12.92g/2g = 6.46 .
sample 2 = mass of lead/ mass of oxygen = 34.27 - 14.39/14.39 = 1.38 .
so, the ratios are not the same.
Hence, the two chemical compounds are not the same, because their ratio is not equal. In both samples the composition of lead and oxygen is different.
To know more about Chemical compounds, check out:
https://brainly.com/question/26487468
#SPJ1
Identify the predominant intermolecular forces in each of these substances.
1. H2O
2. CaCl2
3. CH3CH(CH3)OH
4. CH4
5. NH3
Answers
Answer:
H2O- hydrogen bonding
CaCl2- ion-ion interaction
CH3CH(CH3)OH- hydrogen bonding
CH4- dispersion forces
NH3- hydrogen bonding
Explanation:
Intermolecular forces are secondary bond forces that hold the molecules of a substance together in a given state of matter.
Intermolecular forces account for quite a number of the observed physical properties of a substance such as the boiling and melting point.
If a compound contains hydrogen atom bonded to a highly electronegative element, hydrogen bonding becomes the most dominant intermolecular force, e.g in water and ammonia.
For nonpolar molecules, dispersion forces are the most dominant intermolecular forces. In ionic substance, ion-ion interaction becomes quite prominent.
Name the following lonic Compounds using the lonic naming rules. Remember, place the metal's name
first, followed by the non-metal element, replacing the ending with "-ide"
1.Caci,
2.LIBr
I
3. Bes
4. LIF
5. K Se
6. Sr,P2
7. Baci
8. Feo
9. Fe,
10. CUN
11. Cun,
Please help meeee
Answers
2.Lithium Bromide
3.Beryllium Sulfide
4.Lithium Fluoride
5. Potassium hydroselenide
6. Strontium phosphide
7.Barium Chloride
8.Iron Oxide
9.Iron
10.?
11.Copper Nitride