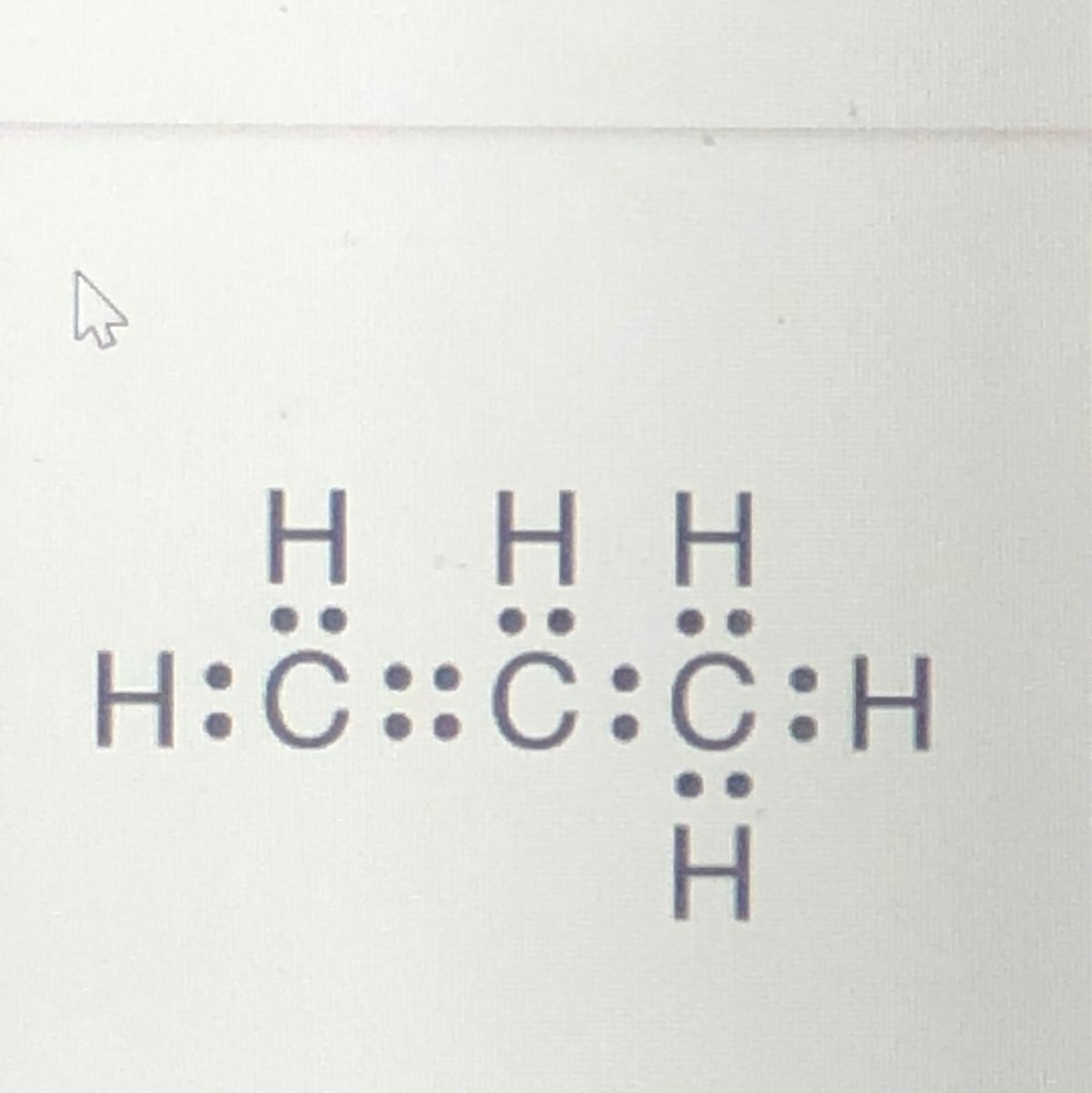
Answers
Answer:
C₃H₆ propene
Explanation:
Find the chemical formula by adding all the carbon and then all the hydrogen together.
Related Questions
in which type of reaction do Pb + O2 form PbO2?
synthesis
double displacement
single displacement
combustion
Answers
Answer: COMBUSTION
Explanation:
A combustion reaction is a reaction of a substance (in this case lead; Pb) with oxygen gas (O₂). It is usually an exothermic reaction that produces an oxide..
Compare the quantiies in each pair.
Comparisons:
=
<
>
a. 50 mL _____ 5 dL
b. 100 mg ____ 10 μg
c. 5 cm ____ 500 mm
d. 10 Ms ____ 10 ms
Answers
a. 50 mL < 5 dL (1 dL = 100 mL)
b. 100 mg > 10 μg (1 mg = 1000 μg)
c. 5 cm > 500 mm (1 cm = 10 mm)
d. 10 Ms > 10 ms (1 Ms = 1000 ms)
To know more about quantities refer to-
brainly.com/question/12986460#
#SPJ11
If you leave your home at noon, and the speed limit is 50 km/h on the highway, what is the approximate time will you get to Atlantic City if Atlantic City is 150 km away? (obeying the speed limit of course)
Answers
Answer:
The time you will get to Atlantic City is 3 pm
Explanation:
Given;
speed limit, v = 50 km/h
distance to be traveled, d = 150km
The time of the motion is given as;
time = distance / speed
t = (150 km) / (50 km/h)
t = 3 hours
Thus, if you leave your home at noon, the time you will get to Atlantic City is given as;
time to get home = 12 pm + 3hours = 3 pm
Therefore, the time you will get to Atlantic City is 3 pm
Someone please help me with this problem:
For the following reaction indicate the oxidation state of each atom in the reactants as well as the oxidation state of each atom in the products. Indicate which atom is being reduced and which is being oxidized. Also, indicate the oxidizing agent and the reducing agent.
2Al (s) + 6HNO3 (aq) -> 2Al(NO3)3 + 3H2
Answers
the mass percent of carbon in pure glucose, c6h12o6, is 40.0 percent. a chemist analyzes an impure sample of glucose and determines that the mass percent of carbon is 38.2 percent. which of the following impurities could account for the low mass percent of carbon in the sample? select one: a. ribose, c5h10o5 b. water, h2o c. sucrose, c12h22o11 d. fructose, c6h12o6, an isomer of glucose
Answers
The carbon mass percent in pure glucose, c6h12o6, is 40.0 percent. A chemist analyses an impure specimen of carbohydrates and defines that the carbon mass percent is 38.2 percent.. Option B - H20 ,water impurities could account for the low mass percent of carbon .
Impurities refer to unwanted substances present in a sample of a pure substance. They can be present in trace amounts or in larger quantities and can affect the properties or performance of the pure substance. Mass percent, also known as weight percent, is a way to express the concentration of a component in a mixture. It is calculated by dividing the mass of the component of interest by the total mass of the mixture, and then multiplying by 100. The resulting number represents the percentage of the total mass that is made up of the component of interest. For example, in the case of glucose, the mass percent of carbon is 40.0% because 40 grams of carbon are present in every 100 grams of glucose.
Learn more about mass percent here:
https://brainly.com/question/15461083
#SPJ4
Convert 55.0 miles per hour to feet per second
Answers
Answer:
80.667
Explanation:
math
What volume of 7.8 M copper (II) sulfate stock solution is needed to prepare 3.25 L of a 5.4 M solution?
WILL MARK BRAINLIEST
Answers
Answer:
The volume of 9.0 M copper (II) sulfate stock solution needed to prepare 3.0 L of a 5.0 M solution is 1.667 L
Explanation:
Dilution is a process by which the concentration of a solute in solution is reduced by adding more solvent.
In other words, dilution is the procedure followed to prepare a less concentrated solution from a more concentrated one, and it simply consists of adding more solvent.
In a dilution the amount of solute does not vary. What varies in a dilution is the volume of the solvent: as more solvent is added, the concentration of the solute decreases, as the volume (and weight) of the solution increases.
The equation used in this case is:
Ci * Vi = Cf * Vf
where
Ci: initial concentration
Vi: initial volume
Cf: final concentration
Vf: final volume
In this case:
Ci: 9 M
Vi: ?
Cf: 5 M
Vf: 3 L
Acetylene gas (C2H2) is produced as a result
of the reaction
CaC2(s) + 2 H2O(ℓ) →
C2H2(g) + Ca(OH)2(aq).
If 37 g of CaC2 are consumed in this reaction, how much H2O is needed?
Answer in units of mol. Answer in units of mol.
Answers
Acetylene gas (C2H2) is produced as a result of the reaction CaC2(s) + 2 H2O(ℓ) → C2H2(g) + Ca(OH)2(aq). If 37 g of CaC2 are consumed in this reaction, 1.1 moles of water needed.
What is mole ?The term mole is defined as the amount of substance of a system which contains as many elementary entities.
1 mole is equal to 6.023 × 10 ²³ molecules.
Given:
mass of CaC₂ = 37 g
mass of water = ?
The balanced reaction is as follows:
CaC₂ + 2H₂O ⇒ C₂H₂ + Ca(OH)₂
2. Convert mass of CaC₂ to moles
molecular mass CaC₂ = 40 + (2 x 12)
= 40 + 24
= 64 g
64 g of CaC₂ = 1 mol
37 g = x
x = (37 x 1) / 64
x = 37/64
x = 0.57 moles of CaC₂
3. Calculate the moles of water
1 mol of CaC₂ ----------------- 2 moles of water
0.57moles of CaC₂ --------- x
x = (0.57 x 2) / 1
x = 1.1 moles of water
Thus, If 37 g of CaC2 are consumed in this reaction, 1.1 moles of water required.
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ1
16. The half-life of carbon-14 is 5,730 years. What fraction of a 1gram
5 points
sample of carbon-14 would remain after 17,190 years? *
O 1/2
O 1/4
O 1/8
O 1/16
Answers
Answer:
i think it 1/2
Explanation:
A feedback mechanism that increases respiration rate to exhale more co2 would be:_________
Answers
As your body needs more oxygen during exercising and produces more carbon dioxide, this might result in a high respiratory rate.
During exercise, your breathing may occur 3–4 times more frequently. Hyperpnea, or rapid, deep breathing, is the body's natural response to increased carbon dioxide generation. The pace of breathing increases along with the blood's concentration of carbon dioxide. The pH of the blood changes dramatically as carbon dioxide levels rise. The chemoreceptors in the medulla oblongata, a part of the hindbrain that controls respiration, detect this shift. To expel the additional carbon dioxide and breathe in more oxygen, the respiratory center speeds up breathing.
Learn more about carbon dioxide here:
https://brainly.com/question/1923482
#SPJ4
A ball rolls from a to point B the total energy of the ball at point a is the same as the sum of its potentlal Energ and kinetic energy at point B which statement best explains the situation 
Answers
UMMM.....I NEED YOUR HELP GUYS....I HAVE GOT A QUESTION FOR YOU.......... Water waves slow down and their wavelength shortens when they reach shallower water.........explain why this happens???????help me out!!
Answers
Answer:
Becausee deep - water waves do not interact with the ocean bottom as they travel , their speed is independent of the water depth . But as waves enter shallow water , interaction with the bottom alters the waves . Wave speed decreases , wavelength shortens and wave height increases .
Hope this helps :)
In a mixture of gases, each gas will exert its own partial pressure, regardless of the partial pressures of other gases in the mixture. 2. In a closed system, pressure and volume have an inverse relationship. 3. The amount of gas that will dissolve in a fluid is determined by the solubility of the gas in the given fluid.
Answers
The statement you provided is correct. In a mixture of gases, each gas exerts its own partial pressure independently of other gases present.
This is known as Dalton's Law of Partial Pressures. Additionally, in a closed system, pressure and volume have an inverse relationship, as described by Boyle's Law.
In a mixture of gases, each gas exerts its own partial pressure: This is known as Dalton's law of partial pressures. According to this law, in a mixture of gases, the total pressure is the sum of the partial pressures of each individual gas in the mixture.
In a closed system, pressure and volume have an inverse relationship: This is known as Boyle's law. According to this law, when the temperature of a gas is held constant, the pressure and volume of the gas are inversely proportional.
The amount of gas that will dissolve in a fluid is determined by the solubility of the gas in the given fluid: This is known as Henry's law. According to this law, the amount of gas that dissolves in a liquid at a given temperature is directly proportional to the partial pressure of the gas above the liquid.
Lastly, the amount of gas that dissolves in a fluid depends on the solubility of the gas in that particular fluid.
To learn more about Dalton's Law here:
https://brainly.com/question/30459983
#SPJ11
Compare and contrast control group and experimental group.
Answers
Answer: An experimental group, also known as a treatment group, receives the treatment whose effect researchers wish to study, whereas a control group does not
Explanation:
The control group and experimental group are compared against each other in an experiment. The only difference between the two groups is that the independent variable is changed in the experimental group. The independent variable is "controlled" or held constant in the control group
help me with chemistry pls. i have no idea anything related to this subject :')

Answers
Question 24:
Answer C : Q is placed in group 13 and period 2.
Question 25:
Answer C : II and IV.
Question 26:
Answer D
Le Chatelier's Principle governs what property?A. Reaction rateB. None of theseC. EquilibriumD. Catalysts
Answers
Equilibrium. Option C is correct
Explanations:What is Le Chatelier's principle?This law states that a new equilibrium state is achieved if the changes in temperature, pressure, concentration and volume will cause a predictable and opposing changes in the system.
This shows that Le Chatelier's principle can be used to predict the properties above to determine the effect equilibrium have on a system.
Based on the above explanations, we can conclude that Le Chatelier's Principle governs the property of Equilibrium.
What is made of only one type of atom?
Answers
Answer:
element
Explanation:
its the only substance type of antom called the element so yeah
A first order reaction has a half - life of 36 min. What is the value of the rate constant? A. 3.2 x 10-4 s-1. B. 1.9 x 10-3 L mol-1 s-1. C. 1.2 s-1
Answers
To determine the rate constant of a first-order reaction, we can use the equation for the half-life of a first-order reaction: t1/2 = ln(2) / k
Given:
Half-life (t1/2) = 36 min We need to convert the half-life from minutes to seconds to match the units of the rate constant. Therefore, t1/2 = 36 min * 60 s/min = 2160 s.
Now we can rearrange the equation and solve for the rate constant (k):
k = ln(2) / t1/2
Substituting the given value, we have:
k = ln(2) / 2160 s Calculating this expression, we find that the rate constant is approximately 3.214 x 10^(-4) s^(-1). Therefore, the correct answer is option A. 3.2 x 10^(-4) s^(-1).
Learn more about first-order reaction here: brainly.com/question/28213237
#SPJ11
A(n) ______ studies weather and the Earth’s atmosphere to predict weather forecasts.
Answers
Answer:
meteorologist
Reactants are the____________ substances in a chemical change and products are the ______substances.
Answers
Answer:
What is the name of the substances that are used at the beginning of a chemical reaction?
The substances that go into a chemical reaction are called the reactants, and the substances produced at the end of the reaction are known as the products.
Explanation: :)
Select all of the statements that correctly describe DNA structure:
Group of answer choices
The pyrimidine and purine bases are located on the outside of the double helix.
The sugars and phosphate groups are located on the exterior of the helix.
Base pairing will always be between a purine base and a pyrimidine base.
Two connected DNA strands will be oriented in the same direction.
Hydrogen bonds will hold two DNA strands together.
Answers
Answer:
Hydrogen bonds will hold two DNA strands together.The sugars and phosphate groups are located on the exterior of the helix.Base pairing will always be between a purine base and a pyrimidine base.I hope this helps you
:)
To ensure that a vehicle crash is inelastic, vehicle safety designers add crumple zones to vehicles. A crumple zone is a part of a vehicle designed to crumple easily in a crash. Use Newton’s second law to explain why crumple zones reduce the force in a collision.
Answers
Answer:
well because with the velocity of the two, using the second law, it can slow the velocity before there is a casualty.
Explanation:
What is a good example of ACCURACY and PRECISION? *
Answers
Answer:
Bow and arrow
Explanation:
A bow and arrow need accuracy to hit a target. It needs precision to hit the small target
NASA shipped 51,300 g of water (H₂O) to the space station. How many grams of Oxygen (0₂) w
at amount of water theoretically produce? Using the balanced equation for electrolysis and mol
asses from Part A and Part B determine how many grams of oxygen (0₂) you will be able to produc
eginning with 51,300 grams of water (H₂O) (3-step grams to moles to moles to grams conversion).
Answers
Answer:Starting with 51,300 grams of water, we can theoretically produce 45,592 grams of oxygen using electrolysis, based on the balanced equation 2H₂O → 2H₂ + O₂.
in the distillation of a pure material, why does all of the pure material no vaportize once the boiling point is reched.
Answers
In the distillation of a pure material, all of the pure material not vaporize once the boiling point is reached because more heat would need to be added to the distillate in order to vaporize the liquid from its boiling point.
During distillation, the process of vaporizing a liquid and collecting the resulting vapor as a purified substance, it is important to consider the energy requirements involved.
When a liquid reaches its boiling point, it undergoes a phase change from the liquid phase to the gas phase. This phase change requires the input of energy in the form of heat. The heat breaks the intermolecular forces holding the liquid molecules together, allowing them to transition into the gas phase.
The heat required to vaporize a liquid is not solely determined by the boiling point. The heat required to convert a liquid into a gas is known as the heat of vaporization, and it varies depending on the substance.
When distilling a liquid, such as water, the heat of vaporization must be supplied to convert the liquid into vapor. This energy is absorbed by the liquid, and it is essential to provide continuous heating to maintain the distillation process.
As the liquid is heated and reaches its boiling point, vaporization begins. However, the rate at which the liquid vaporizes depends on the amount of heat being supplied. If the heat input is insufficient, the vaporization process will be slower, and not all of the liquid will vaporize at once.
To ensure the complete vaporization of a liquid during distillation, a sufficient amount of heat must be continuously applied to the system. This allows the heat of vaporization to be continually supplied to the liquid, facilitating the conversion of the entire liquid into vapor.
If the heat input is insufficient, the vaporization process will be slower, and the liquid may not vaporize all at once. Providing adequate and continuous heating is crucial to ensure the complete conversion of the liquid into vapor during distillation.
To know more about distillation here
https://brainly.com/question/31829945
#SPJ4
the state that is reached when ions are entering solution at the same rate at which they leave solution to regenerate a solid
Answers
The state that is reached when ions are entering solution at the same rate at which they leave solution to regenerate a solid is called solubility equilibrium.
Equilibrium is the state in a chemical system where the forward and reverse reactions occur at equal rates, resulting in no net change in the concentrations of reactants and products. In the context of ions entering and leaving a solution, this refers to a dynamic balance between the dissolution of a solid and the precipitation of the same solid.
When ions are introduced into a solution, they have the potential to dissolve and form hydrated ions, increasing the concentration of the ions in the solution. However, at the same time, the hydrated ions can also combine and precipitate as a solid, reducing the concentration of the ions in the solution. When the rate of dissolution and precipitation becomes equal, the system reaches a state of equilibrium, where the concentration of the ions remains constant over time.
In this equilibrium state, the solution and the solid are in a dynamic balance, with ions continuously entering and leaving the solution, but the overall concentration of the ions remains constant. This concept is fundamental in understanding chemical reactions and the behavior of solutions.
To learn more about solubility equilibrium, here
https://brainly.com/question/17330140
#SPJ4
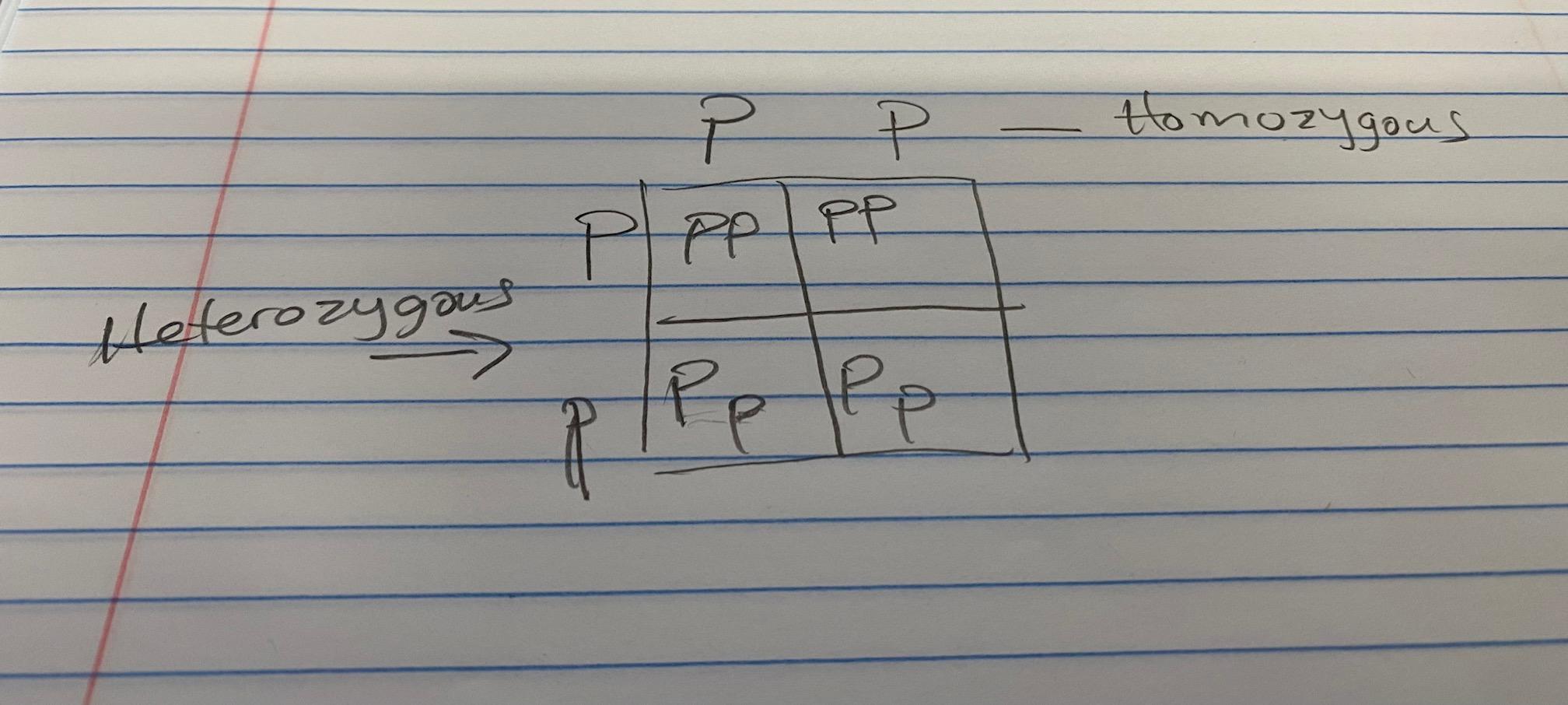
This is the picture btw because it wont let me say all the words.

Answers
Answer:
see image
D is the answer
Explanation:
see image
the box is like a mini multiplication table
20.What is it called when particles in a solid or liquid move slower and closer
together?
Answers
Answer: freezing
Explanation:
when the atoms or molecules in a solid or liquid are moving slower and closer together, it is called brrrrrrrrr freezinf
. describe how the ph of a solution relates to the hydrogen ion concentration. does a solution at ph 1 have more or less hydrogen ions than a solution at ph 4?
Answers
A solution at pH 1 has more hydrogen ions than a solution at pH 4. The pH of a solution refers to the hydrogen ion concentration.
The concentration of hydrogen ions and the pH of a solution are inversely proportional. This means that the higher the hydrogen ion concentration, the lower the pH, and vice versa.
A solution at pH 1 will have more hydrogen ions than a solution at pH 4.The pH of a solution is defined as the negative logarithm of the hydrogen ion concentration. The equation for calculating the pH of a solution is given as follows:
\($$pH = -\log_{10}[H^+]$$\)
In this equation, [H⁺] is the hydrogen ion concentration in moles per liter (mol/L) of solution. A change of 1 pH unit corresponds to a 10-fold change in the hydrogen ion concentration.
Therefore, if a solution has a pH of 1, it has a hydrogen ion concentration of 0.1 mol/L.
If a solution has a pH of 4, it has a hydrogen ion concentration of 0.0001 mol/L. Thus, a solution at pH 1 has more hydrogen ions than a solution at pH 4.
To know more about pH, refer
https://brainly.com/question/15265711
#SPJ11
PLEASE ANSWER!!! 30 POINTS!!!!
The limiting reactant O2 form 2.7 mol AI2O3.
What mass of AI2O3 forms knowing the molar mass of AI2O3 is 102 g/mol?
g AI2 O3
Answers
Answer: The mass of Al2O3 that forms is 275.4 g. Don't worry! Help has arrived! Read the explanation below:
Brainliest?
Explanation:
The balanced chemical equation for the reaction between aluminum (Al) and oxygen (O2) to form aluminum oxide (Al2O3) is:
4 Al + 3 O2 → 2 Al2O3
According to the problem, we know that the limiting reactant is O2 and that it forms 2.7 mol of Al2O3. We can use the stoichiometry of the balanced chemical equation to calculate the amount of Al2O3 that would be formed from 3 mol of O2, which is the amount that would react with 4 mol of Al:
4 Al + 3 O2 → 2 Al2O3
3 mol of O2 → 2 mol of Al2O3
We can use the mole ratio from the balanced equation to convert the amount of O2 that reacted to the amount of Al2O3 that formed:
2.7 mol of Al2O3 × (3 mol of O2 / 2 mol of Al2O3) = 4.05 mol of O2
This tells us that if we had 4.05 mol of O2, it would react completely with 4 mol of Al to form 2.7 mol of Al2O3. However, since we only have a limited amount of O2 (the limiting reactant), we know that not all of the Al will react, and some of it will be left over.
To calculate the mass of Al2O3 that forms, we can use the amount of O2 that reacted (which we just calculated) to determine the amount of Al that reacted:
4 Al + 3 O2 → 2 Al2O3
4.05 mol of O2 × (4 mol of Al / 3 mol of O2) = 5.4 mol of Al
This tells us that 5.4 mol of Al reacted with the 2.7 mol of Al2O3 that formed. To calculate the mass of Al2O3, we can use the mole ratio from the balanced equation and the molar mass of Al2O3:
2.7 mol of Al2O3 × (102 g/mol) = 275.4 g of Al2O3
Therefore, the mass of Al2O3 that forms is 275.4 g.
The balanced chemical equation for the reaction between aluminum (Al) and oxygen (O2) to form aluminum oxide (Al2O3) is:
4 Al + 3 O2 → 2 Al2O3
From the problem statement, we know that O2 is the limiting reactant, which means that all of the Al will be consumed and the amount of Al2O3 that forms will be determined by the amount of O2 available.
We can use the stoichiometry of the balanced equation to relate the amount of O2 to the amount of Al2O3 that forms:
3 mol O2 = 2 mol Al2O3
Therefore, the number of moles of Al2O3 that forms can be calculated as follows:
2.7 mol Al2O3 = (3 mol O2 / 2 mol Al2O3) * x mol O2
where x is the number of moles of O2 that reacts. Solving for x, we get:
x = (2.7 mol Al2O3) * (2 mol Al2O3 / 3 mol O2) = 1.8 mol O2
Now that we know the number of moles of O2 that reacts, we can use the molar mass of Al2O3 to calculate the mass of Al2O3 that forms:
mass of Al2O3 = (1.8 mol O2) * (2 mol Al2O3 / 3 mol O2) * (102 g/mol Al2O3) = 122.4 g
Therefore, the mass of AI2O3 that forms when O2 is the limiting reactant is 122.4 g.