What color is a mirror?
Answers
Answer:
mirrors do not have a color, many people believe they are green because they reflect the most green light back into our eyes.
Related Questions
Isotopic Atomic Atomic Number Number Number Charge
Notation Number
Mass
of
of
of
Number Protons
Neutrons
Electrons
18
CI
27 A1+1
N-15
17
45
101
43
-1

Answers
Atoms of different element can not have same atomic number because only same type of atoms combine to form element. Atoms belonging to different element can have different atomic number. Therefore, blanks can be filled as below.
What is element?Element generally consist of atoms or we can atoms combine to form element. Atoms of an element is always same, means all the properties of all atoms of one type of element is same. Two or more than two atoms with different physical or chemical properties can not combine together to form an element.
isotopic Atomic atomic mass number of number of number charge
notation number number protons electrons neutrons
Cl 17 35 17 17 18 0
Al⁺¹ 13 27 13 13 14 +1
Rh 45 101 45 45 56 0
N-15 7 15 7 7 8 0
Therefore, blanks can be filled as above.
To know more about element, here:
https://brainly.com/question/8460633
#SPJ1
Which one of these have the biggest ionization energy and why? O, N, F
Answers
Answer: Fluorine has the highest ionization enthalpy.
Explanation: This is because the ionization energy decreases from top to bottom and increases from left to right. Also, ionization enthalpy is the amount of energy required by an isolated gaseous atom to lose an electron in its ground state.
Since fluorine needs just one atom to attain noble gas configuration so it will not want to lose any electron and hence a high amount of energy will be required to remove an electron from it.
What quantity in moles of CaF2 are in 75.5G of CaF2?
Answers
Considering the definition of molar mass, the amount of moles of CaF₂ in 75.5 g of CaF₂ is 0.968 moles.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it multiplied by the number of times they appear in the compound.
Molar mass of CaF₂In this case, you know the molar mass of the elements is:
Ca= 40 g/moleF= 19 g/moleThe molar mass of the compound CaF₂ is calculated as:
CaF₂= 40 g/mole + 2× 19 g/mole
Solving:
CaF₂= 78 g/mole
Moles of 75.5 g of CaF₂You can apply the following rule of three: If by definition of molar mass 78 grams of the compound are contained in 1 mole, 75.5 grams of the compound are contained in how many moles?
moles= (75.5 grams× 1 mole) ÷78 grams
moles= 0.968 moles
Finally, the amount of moles is 0.968.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
starvation would cause which of the following acid-base conditions? also, determine what type of compensation (metabolic or respiratory) there would be.A. respiratory acidosis with metabolic compensation B. respiratory alkalosis with metabolic compensationC. metabolic acidosis with respiratory compensation D. metabolic alkalosis with respiratory compensation
Answers
In the below acid-base conditions, starvation will cause severe alkalosis without respiratory correction.
What are the 4 types of respiratory?The helps in proper that make it up respiration are as follows: Pulmonary ventilation is the process of forcing air into and out of the lungs. External respiration is the process through which dioxide and oxygen are transferred from the lung to tissues. internal respiration, cellular gas exchange, and blood flow through systemic capillaries.
What is the main cause of respiratory?Respiratory diseases can be brought on by infections, smoking, exposure to smoke exposure, asbestos, radiation, and other forms of air pollution. Some of respiratory conditions include allergies, chronic obstructive pulmonary disease (COPD), respiratory failure, bronchitis, and lung cancer.
To know more about respiratory visit:
brainly.com/question/15877304
#SPJ4
I just need help on the reactivity series stuff please I need to pass my grade check HELP
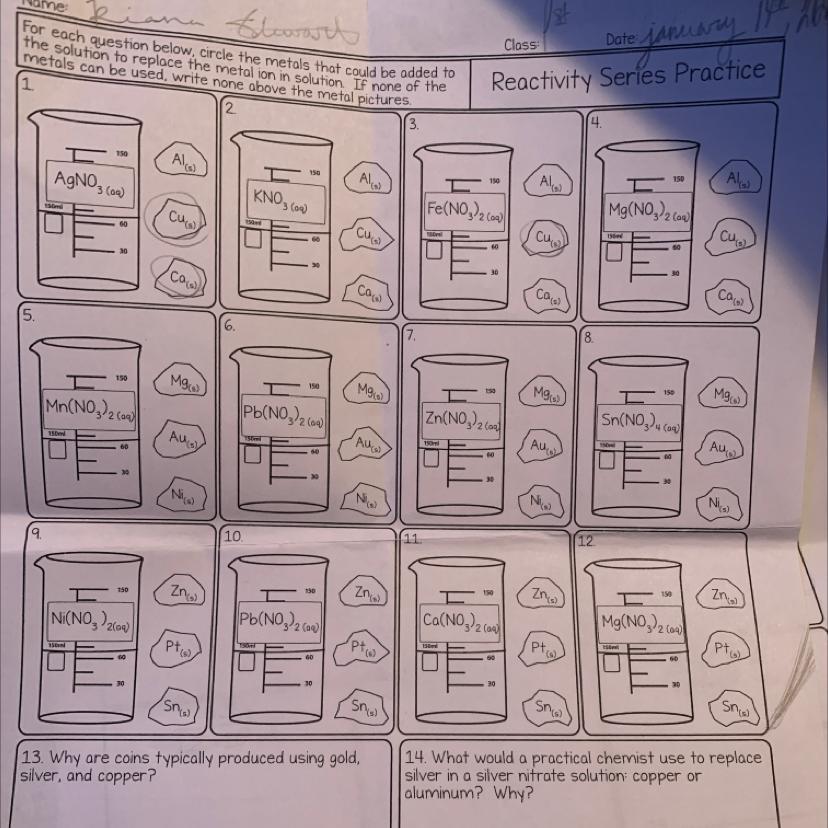
Answers
Reactivity series:-
\(\setlength{\unitlength}{0.8 cm}\begin{picture}(20,15)\thicklines\put(5,9.5){\large {\boxed{ \sf{Reactivity \: series}}}}\put(0.8,8){\large\sf K \qquad\qquad\qquad Potassium\qquad\qquad\qquad \: maximum}\put(0.8,7.2){\large\sf Na \qquad\qquad\qquad Sodium\qquad\qquad\qquad\quad \: reactivity}\put(0.8,6.2){\large\sf Ca\qquad\qquad\qquad \: Calcium}\put(0.8,5.2){\large\sf Mg\qquad\qquad\quad \: Magnesium}\put(0.8,4.2){\large\sf Al\qquad\qquad\qquad \: Aluminium\qquad\qquad\qquad \: decreasing}\put(0.8,3.2){\large\sf Zn\qquad\qquad\quad\qquad \: Zinc\qquad\qquad\qquad\qquad \:reactivity}\put(0.8,2.2){\large\sf Fe\qquad\qquad\quad\qquad Ferrous}\put(0.8,1.2){\large\sf Pb\qquad\qquad\qquad \: \:\: Lead}\put(0.8,0.2){\large\sf H\qquad\qquad\qquad \: \:Hydrogen}\put(0.8, - 0.6){\large\sf Cu\qquad\qquad\qquad \: Copper}\put(0.8, - 1.5){\large\sf Hg\qquad\qquad\qquad \: Mercury}\put(0.8, - 2.5){\large\sf Ag\qquad\qquad\qquad \: \:Silver}\put(0.8, - 3.5){\large\sf Au\qquad\qquad\qquad \: \: Gold\qquad\qquad\qquad\quad \: \: minimum reactivity}\put(9.3,8.2){\vector(0, - 2){12}}\end{picture}\)
#1
Ag is found as too less reactive so
Cu✓Al✓Ca✓#2
No element in universe can displace potassium (K) from its solution as it's most reactive .
Nota#3
Fe is also a reactive metal.
Only Cupper is less reactive than itSo
Al✓Ca✓#4
Only Calcium can displace Mg#5
Mg and Ni can do ,Aurum(Gold) is least reactive so can't#6
Only Mg#7
Only Mg#8
Mg and Ni#9
Only Zn#10
Zn and Pt#11
NotaAs Ca is third most reactive metal
#12
NotaTemperature and volume are
_____ related, which means as temperature______
the volume decreases.
Answers
Answer:
They are directly related.
Temp decrease then volume decrease
Temp increase then volume increase
differentiate between edible and non edible mushroom
Answers
Edible mushrooms: Consuming edible mushrooms is safe and provides health advantages like fiber, vitamins, and minerals.
Examples: Button mushrooms.Non-Edible mushrooms: Mushrooms that cannot be eaten could be harmful or have unappealing flavors and textures that could be harmful if consumed.
Examples: Death Cap.
Metal carbonyls, which have the general formula M(CO)x (x can be various possible subscripts depending on the particular metal used), are important intermediates in the purification and reactivity of transition metals. Consider the following synthesis of an unknown metal carbonyl, with the indicated equilibrium constant. M (s) + x CO (g) --> M(CO)x (g) Kp = 0.133 If you add an unknown amount of the metal and carbon monoxide gas to an empty reaction flask, and at equilibrium the pressure of carbon monoxide is 2.28 atm and metal carbonyl is 7.80 atm, what was the initial pressure of carbon monoxide that you added?
Answers
Metal carbonyls are characterized as volatile, low-melting-point compounds. They are created from the compound Mx(Co)y, which upon heating breaks down into metal and carbon monoxide. Contact with the skin can make them toxic.
In some cases, such as in nickel tetracarbonyl (Ni(CO)4), these complexes are homoleptic and only contain CO ligands, but more frequently, metal carbonyls are heteroleptic and contain a variety of ligands. Mononuclear metal carbonyls have a single metal atom at their core.
Learn more about the Mononuclear
brainly.com/question/28304567
#SPJ4
Please help fast
1. What do the states at room temperature and the melting points tell you about the strengths of the forces between the particles for each of the three substances?
2. Room temperature is approximately 20°C for scientific purposes. A hot pan on a stove can reach temperatures up to about 260°C. Given this information, what can you conclude about the melting points of sugar and salt?
3. Consider the melting points of sugar and salt, as well as the states of sugar, salt, and oil at room temperature. What can you conclude about the attractive forces between particles in the three substances?
Answers
1. The states of the three substances and the strengths of the forces between the particles for each of the three substances are described as follows:
sugar- solid state; strong attractive forces between the particles.salt- solid state; strong attractive forces between the particles.oil- liquid state; weaker attractive forces between the particles.2. Given the information on room temperature and the temperature of the hot pan stove, it can be concluded that the melting point of sugar is less than the melting point of salt.
3. Considering the melting points of sugar and salt, as well as the states of sugar, salt, and oil at room temperature, the nature of the attractive forces between particles in the three substances can be described as follows:
salt - has the highest attractive forces between its particlessugar - has the next highest attractive forces between its particlesoil - has the lowest attractive forces between its particlesWhat are the attractive forces between particles?The attractive forces between particles of a substance are the forces that exist between the particles of the substance that keep the particles of the substance close to each other.
The stronger the attractive forces between particles of a substance, the more orderly the structure of the substance will be.
Solids have the highest attractive forces between their particles
Liquids - have the next highest attractive forces between their particles
gases - have the lowest attractive forces between their particles
Learn more about attractive forces in a substance at: https://brainly.com/question/8363662
#SPJ1
Sorry for the spamming, but this question is URGENT here,
Which of the following is
property of metals?
A. brittle
B. good conductivity."
C. poor conductivity
(Btw here’s a picture :O)

Answers
Option B, Metals have good conductivity.
Hope it helps you.. pls mark brainliest if it helps you
Is freezing point depression a colligative property?
Answers
Answer:
Yes it is a colligative Property
Explanation:
There is freezing point depression, boiling point elevation, Osmotic Pressure, Vapor Pressure Lowering
The radioactive isotope used for carbon dating is
1. Carbon 12
2. Carbon 13
3. Carbon 15
4. Carbon 14
Answers
Carbon 14 would be your answer, however if it's more than one answer it would also be Carbon 12, so yeah. Your best bet would be Carbon 14.
3 points question 30 which best describes carbon sequestration? the process of removing co2 from the atmosphere and storing it underground or in biomaterials (trees etc) the process of capturing co2 and releasing it into space the process of collecting solid carbon and burying it deep underground the process of mining carbonate rocks and relesing their co2 into the atmosphere
Answers
The best description of carbon sequestration is that it is the process of removing CO2 from the atmosphere and storing it underground or in biomaterials such as trees and plants. This corresponds to option a.
Carbon sequestration plays a vital role in mitigating climate change by reducing the concentration of CO2, a greenhouse gas, in the atmosphere.
Through various methods such as reforestation, afforestation, and carbon capture and storage (CCS) technologies, CO2 is captured and stored long-term, preventing its release into the atmosphere.
Storing CO2 underground involves injecting it into geological formations like depleted oil and gas reservoirs or deep saline aquifers.
Additionally, plants absorb CO2 through photosynthesis, converting it into biomass, which can be stored in forests, soils, and other organic materials.
Carbon sequestration offers a potential solution to help offset anthropogenic carbon emissions and limit their impact on the climate system, contributing to the global efforts to combat climate change. This corresponds to option a.
To know more about carbon sequestration, refer here:
https://brainly.com/question/31439038#
#SPJ11
the mass of a calibrated flask is 25.0 g when empty, 75.0 g when filled with water, and 88.0 g when filled with glycerin. find the specific gravity of glycerin.
Answers
The specific gravity of the glycerin, given that the mass of a calibrated flask is 25.0 g, is 1.26
How do I determine the specific gravity of the glycerin?First, we shall determine the volume of the caliberated flask by obtaining the volume of the water. This shown below:
Mass of caliberated flask = 25 gMass of caliberated flask + water = 75 gMass of water = 75 - 25 = 50 gDensity of water = 1 g/mLVolume of water =?Volume = mass / density
Volume of water = 50 / 1
Volume of water = 50 mL
Thus, the volume of the caliberated flask is 50 mL
Next, we shall determine the density of the glycerin. This shown below:
Mass of caliberated flask = 25 gMass of caliberated flask + glycerin = 88 gMass of glycerin = 88 - 25 = 63 gVolume of caliberated flask = 50 mLVolume of glycerin = Volume of caliberated flask = 50 mLDensity of glycerin =?Density = mass / volume
Density of glycerin = 63 / 50
Density of glycerin = 1.26 g/mL
Finally, we shall determin the specific gravity of the glycerin. Details below:
Density of water = 1 g/mLDensity of glycerin = 1.26 g/mLSpecific gravity of glycerin =?Specific gravity of glycerin = Density of glycerin / Density of water
Specific gravity of glycerin = 1.26 / 1
Specific gravity of glycerin = 1.26
Learn more about specific gravity:
https://brainly.com/question/28960105
https://brainly.com/question/9895615
#SPJ1
notice that the oncoming wind tends to flatten out each drop as it falls. what's different about liquids x and y? your answer should be the one- or two-word name of a physical property
Answers
This surface to volume ratio of a descending droplet in a gravity pull is what causes the droplets to be spherical. Remember that the volume to surface area ratio of a sphere is low.
What size water droplet is the smallest?
The lowest cluster with a tri structure and nicknamed the tiniest water droplet, the freshwater hexamer (H2O)6, is the subject of research by Richardson et al (3). Its structural properties have been experimentally determined thanks to advanced spectroscopic techniques (4).
How big is a droplet?
A average raindrop measures 2 centimeters (2000 microns) is size, a large aerosols particles is 100 microns across, a tiny aerosol particles is 1 micron across, and a cloud droplet is typically 20 microns across.
To know more about droplet:
https://brainly.com/question/19168421
#SPJ4
Suppose a small amount of heat Q flows from a system A at high temperature (350K) to a system B at low temperature (250K). If Q = 0. 5 J, mA = 1. 2 kg, and mB = 0. 6 kg, what will the total entropy change of the system be as a result?
Answers
A small amount of heat Q flows from a system A at high temperature (350K) to a system B at low temperature (250K). If Q = 0. 5 J, mA = 1. 2 kg, and mB = 0. 6 kg, the total entropy change of the system be 0.00057J/K.
Firstly we will be taking Q as a positive number,
Then, the entropy lost by A is Q/TA
= (-0.5 J)/(350 K)
= -1/700 J/K
= -0.00143 J/K.
Now, the entropy gain by B is Q/TB
= (0.5 J)/(250 K)
= 1/500 J/K
= +0.00200 J/K.
Therefore, the total entropy change of the system will be the the sum of entropy lost by A and entropy gain by B.
Hence the total entropy would be =0.00057 J/K.
To know more about entropy refer to-
brainly.com/question/6364271#
#SPJ4
Al + FeCl2 → Fe + AlCl3.
Answers
Answer:
Hope this helps :)AlCl3 + Fe = Al + FeCl2
Chemical Equation Details aluminum chloride + iron = aluminum + iron(ii) chloride
\(\text{Balanced reaction:}\\\\2\text{Al} + 3\text{FeCl}_2 \longrightarrow 3\text{Fe} + 2\text{AlCl}_3\)
Explain what makes a cirrus cloud.
Answers
Answer:
the answer is below
Explanation:
Cirrus clouds form from the ascent of dry air, making the small quantity of water vapour in the air undergo deposition into ice (to change from a gas directly into a solid). Cirrus is made up completely of ice crystals, which provides their white colour and form in a wide range of shapes and sizes.
Atoms with a positive or negative charge are called.
Answers
Answer:
Ions.
Explanation:
An ion is any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations and negatively charged ions, anions.
Atoms with a positive or negative charge are called as ions. If the atoms possess positive charge it is known as a cation, if the atoms possess a negative charge it is known as an anion.
An ion is any atom or group of atoms that bears one or more positive or negative electrical charges.
A positive or negative electric charge is present in an atom or group of atoms due to the loss or gain of one or more electrons (such as a free electron). Having a net electrical charge makes an atom or molecule an ion. By convention, the charge of an electron is thought to be negative, and this charge is equal to and the opposite of the charge of a proton, which is thought to be positive.
A positively charged ion is a cation (atoms or groups of atoms that have more protons than electrons due to having lost one or more electrons). Negatively charged ions are known as anions (meaning they have more electrons than protons due to having gained one or more electrons).
To know more about ions here
brainly.com/question/4933048
#SPJ2
The question should be
What are Atoms with a positive or negative charge called?
if the temperature of a radiator is increased from 27ºc to 54ºc, by what factor does the radiating power change?
Answers
The power radiated by the radiator changes by a factor of 14.1573 when the temperature is increased from 27°C to 54°C.
The formula that relates the power radiated by an object with the fourth power of the temperature is known as the Stefan-Boltzmann Law. It is stated as follows: P = σA(T⁴), where P is the power radiated, σ is the Stefan-Boltzmann constant (5.67 × 10⁻⁸ W/m²K⁴), A is the surface area of the radiator, and T is the temperature in Kelvin.
We must first convert the temperature to Kelvin:
TK = T°C + 273.15
TK1 = 27°C + 273.15 = 300.15 K
TK2 = 54°C + 273.15 = 327.15 K
The factor by which the power radiated changes is the ratio of the power at the new temperature to the power at the original temperature. The equation is as follows:
P2/P1 = (T2/T1)⁴
Substituting the given values:
P2/P1 = (327.15/300.15)⁴
P2/P1 = 1.8856⁴
P2/P1 = 14.1573
You can learn more about the temperature at: brainly.com/question/7510619
#SPJ11
Analyze Data In the figure to the left, write the charge of the oxygen atom and the oxygen ion. Write the charge to the left of the word "charge."

Answers
The image that is shown is the gain of two electrons by the oxygen atom.
What is an ion?We have to note that when we talk about the term "ion" we are talking about such a specie that can be formed by the loss or gain of electrons. Thus an ion is positive if it formed by the loss of electrons and the ion is negative if it is formed by the gain of electrons.
In the case of the image that we have here, we can see that there is again of two electrons by the oxygen atom and this is how we form the oxygen ion that has ten electrons and eight protons.
Learn more about ions:https://brainly.com/question/29183072
#SPJ1
A compound, X contains 92.31 % C and 7.69 % H.
RMM of X is 78. Determine the molecular formula.
Answers
Answer:
C6H6
Explanation:
Just use the table method to find the ratio of C to H and then compare the RMM with that of the empirical formula.

Answer:
C6H6
Explanation:
We divide each percentage by the relative atomic mass of the element:
Carbon: 92.31 / 12.011 = 7.68
Hydrogen: 7.69 / 1.008 = 7.63
So the ratio of carbon to hydrogen is 1:1.
Empirical formula is CH - which has a MM of 13.019
The RMM is 78 so the molecular formula = 78/13.019 = 6 * CH
So it is C6H6 (Benzene).
For the following questions, use the reaction N02(0) -> JN2(9) + 02(9), with AH = -33.1 kJ/mol
and AS = 0.06302 kJ/(mol K).
Draw a possible potential energy diagram of the reaction. Label the enthalpy of the reaction.
Answers
The potential energy diagram for the reaction N02(0) -> JN2(9) + 02(9) should be a curve that descends from reactants to intermediates and then ascends to products, with a label indicating that ΔH = -33.1 kJ/mol.
A potential energy diagram of a reaction is a graph that demonstrates the progress of a reaction and the changes in potential energy that occur over time. The vertical axis of the diagram shows potential energy, and the horizontal axis shows reaction progress. Reactants are shown on the left, intermediates are shown in the middle, and products are shown on the right. The reaction pathway is depicted with a curve that descends from reactants to intermediates and then ascends to products. Labeling the enthalpy of a reaction involves identifying its enthalpy change. The enthalpy change (ΔH) of a reaction is the heat absorbed or given off by the system at a constant pressure. The reaction N02(0) -> JN2(9) + 02(9) has ΔH = -33.1 kJ/mol. The reaction is exothermic because the enthalpy change is negative.
The potential energy diagram for this reaction should be a curve that descends from reactants to intermediates and then ascends to products. The enthalpy change should be labelled in the diagram. If the enthalpy change is negative, then it should be labelled as a negative value. If the enthalpy change is positive, then it should be labelled as a positive value.
for such more questions on energy
https://brainly.com/question/29339318
#SPJ8
7.
How many significant figures are in the number .0030?
a.1
b. 2
c. 3
d. 4
e. 0
Answers
Explanation:
any zero coming last after any decimal point is not recognized as a number hence .0030 is equivalent .003 therefore there is only one significant figures in the number .0030
a. 1
The scale on the front panel of a certain manual spectrophotometer reads from 0.0010 to 100 % transmittance. What are the corresponding absorbance values at these two ends of the scale?
Answers
The relationship between transmittance (T) and absorbance (A) is given by the equation:
A = -log10(T)
To find the corresponding absorbance values at the two ends of the scale, we can substitute the transmittance values into the equation.
For 0.0010 % transmittance:
A = -log10(0.0010)
For 100 % transmittance:
A = -log10(1)
Since the log of 1 is 0, the absorbance at 100 % transmittance is 0.
Calculating the absorbance values for the given transmittance values will provide the corresponding values at the two ends of the scale.
Know more about transmittance here;
https://brainly.com/question/15692537
#SPJ11
2.
Si la masse volumique d'une substance est 10g/ml et sa masse est 80g, quel est
son volume??
Answers
Answer:
C
Explanation:
how many milliliters of a stock solution of 5.00 mm hno3hno3 would you have to use to prepare 0.200 ll of 0.550 mm hno3hno3 ?
Answers
You would have to use 11 milliliters of the stock solution of 5.00 mm HNO3 to prepare 0.200 L of 0.550 mm HNO3.
To calculate the volume of the stock solution required, we can use the formula:
C1V1 = C2V2
Where C1 is the concentration of the stock solution, V1 is the volume of the stock solution needed, C2 is the desired concentration of the final solution, and V2 is the final volume of the solution.
In this case, the concentration of the stock solution (C1) is 5.00 mm HNO3 and the desired concentration of the final solution (C2) is 0.550 mm HNO3. The final volume of the solution (V2) is 0.200 L.
Plugging these values into the formula, we have:
(5.00 mm HNO3)(V1) = (0.550 mm HNO3)(0.200 L)
Solving for V1, we get:
V1 = (0.550 mm HNO3)(0.200 L) / (5.00 mm HNO3)
V1 = 0.011 L
To convert this volume to milliliters, we multiply by 1000:
V1 = 0.011 L * 1000 mL/L
V1 = 11 mL
Therefore, you would have to use 11 milliliters of the stock solution of 5.00 mm HNO3 to prepare 0.200 L of 0.550 mm HNO3.
Learn more about stock solution here:-
https://brainly.com/question/33305064
#SPJ11
40 points. Explain how a substituted hydrocarbon is made.
Answers
Answer: Organic acids form when a carboxyl group (−COOH) is substituted for one of the hydrogen atoms in a hydrocarbon.
you created a standard curve and calculated the slope to be 64.52. after synthesizing your copper network, you weighed 0.059 grams of your copper product, worked it up with nitric acid, buffer, and ammonia and added it to a 25 ml volumetric flask. then, you measured the absorbance of your solution and received a value of 0.374. what is the % mass of copper in your product?
Answers
The % mass of copper in your product will be 3.93 %.
Calculated slope = 64.52
Mass of copper product = 0.059 g
Initial volume (V1) = 25 ml
Final volume (V2) = 100 ml
Absorbance (A) = 0.374
% mass of copper = ?
To find out the concentration (C) we use the following equation
C = A / slope
Put the values
C (M2) = 0.374 / 64.52
C (M2) = 0.005797 g/L
To calculate M1 we use the following equation.
M1V1 = M2V2
M1 = 0.005797 * 100 ml / 25 ml
M1 = 0.0232 g/L
mass of copper = Penny solution x Volume
mass of copper = 0.0232 g/L x 0.1 L
mass of copper = 0.00232 g
% mass of copper = (total mass of copper ÷ mass of penny) x 100
% mass of copper = (0.00232/ 0.059 ) x 100
% mass of copper = 3.93 %
You can also learn about percent mass from the following question:
https://brainly.com/question/5394922
#SPJ4
Hypothesis:
Before you begin, predict how many compounds you can make from the elements below. Pick one element from the metal list and two from the nonmetal list.
For example, "If I use sodium, hydrogen, and oxygen, then I can make ten new compounds in five minutes."
Now, you try! Make a prediction. Be sure to record your prediction on your lab report.
Metal Nonmetal
• Sodium (Na)
• Calcium (Ca)
• Hydrogen (H)
• Oxygen (O)
• Carbon (C)
• Chlorine (Cl)
Answers
I can form two compounds if I combine calcium, hydrogen and oxygen in five minutes.
What is a compound?A compound is formed by the combination of atoms. It is important to note that the same atoms can be combined in different ways to produce new compounds.
I can form two compounds if I combine calcium, hydrogen and oxygen in five minutes.
Learn more about compounds:https://brainly.com/question/13516179?
#SPJ1