Answers
Answer:
what is matter the be use
Related Questions
An clement X has 2 electrons in K shell, 8 electrons in L shell and 5 electrons in i Size of X ion is greater than that of X atom though both contain the same protons. Give reason. ii) Write down the formula of one of the compounds of X where X is in -3 oxidation.
Answers
Answer:
i) The size of X ion is greater than that of X atom even though both contain the same number of protons because the ion has fewer electrons compared to the atom. When an atom forms an anion (negative ion), it gains electrons, which causes increased electron-electron repulsion. This repulsion causes the electron cloud to expand, and as a result, the ion becomes larger than the neutral atom.
In the case of element X, when it forms an ion with a -3 charge, it will gain 3 more electrons, increasing the total number of electrons to 18. This will cause the size of the X ion to be larger than the neutral X atom.
ii) To determine the compound of X in the -3 oxidation state, we first need to determine the element's identity. We know that X has 15 electrons in total (2 in the K shell, 8 in the L shell, and 5 in the M shell). Therefore, X has an atomic number of 15, which corresponds to phosphorus (P).
Since phosphorus is in the -3 oxidation state, it gains 3 electrons and becomes P^3-. To form a compound, we need a cation that can balance the negative charge. A common example is aluminum (Al), which has a +3 charge (Al^3+). When phosphorus and aluminum combine, they form the compound aluminum phosphide with the formula AlP.
Define pressure. Group of answer choices force exerted by solids to the surrounding area force used to compress a gas force used to melt a solid force exerted per unit area by gas particles as they strike the surfaces around them force applied to a gas to condense it
Answers
Answer:
force exerted per unit area by gas particles as they strike the surfaces around them
Explanation:
According to the kinetic molecular theory, a gas is composed of molecules. The molecules of a gas are in constant random motion and collide frequently with each other as well as with the walls of the container.
Pressure is defined as force per unit area. The pressure of a gas is the force exerted per unit area by gas particles as they strike the surfaces around them hence the answer above.
One day it is clear and sunny, but you notice that the pressure is less than it was the day before. What weather might be coming? Why?
Answers
Answer:
I believe your answer is
Explanation:
when the pressure is less I believe it means rain is coming
Identify all the signs of a chemical change in the description below:
A clear liquid is placed in a beaker and heated. As the liquid is heated, bubbles form and, gradually, the liquid decreases in the container.
Answers
Answer:
When a substance undergoes changes in its chemical composition or combines with another substance to form a new substance is referred to as chemical change. In contrast, physical change is defined as the change in appearance or state of matter and not the chemical composition of the substance.
Explanation:
When a clear liquid is boiled or heated, the kinetic energy of the molecules increases, and the evaporation of the liquid takes place faster. For example, evaporation is a type of physical change, in which the liquid is heated to form bubbles or vapors. These vapors rise in the air and decrease the volume of the liquid. In this process, the liquid has only changed its state of matter (liquid to gas) and not the chemical composition. Thus, there is no chemical change taking place in the heating of water and only physical change is taking place.
Cho các quá trình và số liệu sau:
C(d,s) ⭢ C (g,s) (1) : ΔH0298 = - 1,9 kJ
ΔG0298 = - 2,87 kJ
C(g,s) + O2 (k) ⭢ CO2 (k) (2): ΔH0298 = - 393,5 kJ
a)Giải thích tại sao trạng thái chuẩn của C lại là C(g) mà không phải là C (d).
b)Tính ΔH0298 của phản ứng sau:
C(d,s) + O2 (k) ⭢ CO2 (k) (3)
ΔH0298 của phản ứng 3 có phải là ΔH0298, sinh nhiệt của CO2 không ? Tại sao?
Answers
Answer:
rrgggf in the world of the world of the world of the up the good morning I will take a user and pass it on the
25020
Oriol
250g
The reaction is in equilibrium in a closed container. In this state N, gas is introduced in the
container which does not react with the reactants and products..
1. Analyze the change of the equilibrium in the reaction.
2x2=4
i. Keeping the volume of the container constant
11. Keeping the pressure constant
2. If at equilibrium reaction mixture contains 30% mole SO: of so, what is the maximum
number of SO: molecules produced from 10 mole SO, and 5 mole O?
2.
3. Will there be any change in equilibrium if water is added to the reaction
container? Give your
opinion
1
4. Is it possible to prepare any buffer solution by adding water first and then alkali in the
reaction container? Analyze
3
Answers
Using the following equation:
2 NaOH + H2SO4 à 2 H2O + Na2SO4
How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you have an excess of sulfuric acid?
Answers

how do protons neutrons and electrons affect the overall charge of an atom
Answers
Answer:
It determines the stability electric state of the atom
Explanation:
Now atoms are stable depending on the number of neutrons they have in their nuclei. If the no of electrons Equal no of protons then the atom is said to be neutral
bear in mind that neutrons have no charge so they don't affect the atom's electric state.
it's the neutrons that determines the atom's radioactive state
Which set of products is correct for
this double replacement reaction?
2KI(aq) + Pb(NO3)2(aq) -
Remember that each formula must have a
balanced charge.

Answers
After considering all the given options we come to the conclusion that the set of products that are accurate for double replacement reaction is 2KNO₃ (aq) + PbI₂ (s), which is Option D under the condition that the given reaction is 2KI(aq) + Pb(NO₃)₂(aq).
A double replacement reaction generally comprised of reactions in regarding the positive and negative ions of two ionic compounds exchanges locations to form two new compounds. The general form for this type reaction is: AB + CD → AD + CB .
For instance, when silver nitrate (AgNO₃) is added with sodium chloride (NaCl), the following reaction takes place AgNO₃ + NaCl → AgCl + NaNO₃ ²
The correct set of products for the double replacement reaction 2KI(aq) + Pb(NO₃)₂(aq) is 2KNO₃ (aq) + PbI₂ (s).
To learn more about double replacement reaction
https://brainly.com/question/27941291
#SPJ1
Classify each element.

Answers
A material that has been treated, pοlished, οr brοken recently has a shiny appearance and is excellent at transferring heat and electricity. Metal is the name. Metals are typically flexible and ductile.
What dοes ductile mean tο yοu?A material's ductility is its capacity tο withstand being hammered thin οr stretched intο wire withοut breaking.
In: Pοst-transitiοn metals include indium. At rοοm temperature, it remains sοlid.
Rn: Radοn, is delegated a respectable gas. At rοοm temperature, it is gas.
Se: Selenium belοngs tο the nοn-metal class. At rοοm temperature, it remains sοlid.
As: Arsenic falls intο the metallοid οr semi-metal categοry.
Ru: Ruthenium belοngs tο the class οf transitiοn metals. At rοοm temperature, it remains sοlid.
Ta: Tantalum belοngs tο the grοup οf transitiοn metals. At rοοm temperature, it remains sοlid.
Eu: Eurοpium belοngs tο the class οf lanthanides. At rοοm temperature, it remains sοlid.
Learn more about ductility:
brainly.com/question/496496
#SPJ1
The six metals have the work functions, E0.A. Rank these metals on the basis of their cutoff frequency.B. Rank these metals on the basis of the maximum wavelength of light needed to free electrons from their surface. C. Each metal is illuminated with 400 nm (3.10 eV) light. Rank the metals on the basis of the maximum kinetic energy of the emitted electrons.1. Cesium: w = 2.1 eV 2. Aliminium: w = 4.1 eV 3. Beryllium: w = 5.0 eV 4. Potassium: w = 2.3 eV 5. Platinium: w = 6.4 eV 6. Magnesium: w = 3.7 eV
Answers
Answer:
a) Platinum > Beryllium > Aluminum > Magnesium > Potassium > Cesium
b) Cesium > Potassium > Magnesium > Aluminum > Beryllium > Platinum
c) Cesium > Potassium
Explanation:
We must recall that the frequency of an electromagnetic wave is directly proportional to its energy. Hence as the work function of the metal increases, the minimum frequency required for emission of electrons occur increases accordingly.
Similarly, the maximum wavelength required for electron emission to occur varies inversely as the work function of the metal hence the answer provided.
Lastly, only caesium and potassium has work function less than the energy of the incident photon hence only these two metals experience electron emission with the kinetic energy of electrons emitted from caesium greater than that emitted from potassium.
Determine the number of moles in each
of the following substances.
1. 67.42 g Si
2. 11.82 g gold
3. 28.8 g Br₂
Answers
To determine the number of moles in each substance, we need to divide the mass of the substance by its molar mass.
67.42 g Si:
The molar mass of Si is 28.0855 g/mol (rounded to 4 decimal places). Therefore, the number of moles of Si is:
67.42 g / 28.0855 g/mol = 2.3992 mol (rounded to 4 decimal places).
11.82 g gold:
The molar mass of gold is 196.9665 g/mol (rounded to 4 decimal places). Therefore, the number of moles of gold is:
11.82 g / 196.9665 g/mol = 0.060 mol (rounded to 3 decimal places).
28.8 g Br₂:
The molar mass of Br₂ is 159.808 g/mol (rounded to 3 decimal places). Therefore, the number of moles of Br₂ is:
28.8 g / 159.808 g/mol = 0.1803 mol (rounded to 4 decimal places).
Therefore, the number of moles in each substance is:
2.3992 mol Si
0.060 mol gold
0.1803 mol Br₂.
each student in a class placed a 2.00 g sample of a mixture of cu and al in a beaker and placed the beaker in a fume hood. the students slowly poured 15.0 ml of 15.8 m hno3(aq) into their beakers. the reaction between the copper in the mixture and the hno3(aq) is represented by the equation above. the students observed that a brown gas was released from the beakers and that the solutions turned blue, indicating the formation of cu2 (aq). the solutions were then diluted with distilled water to known volumes. question which of the following is true about the reaction?
Answers
It is a redox reaction because CU is oxidized and the nitrogen atom in NO3 is reduced.
A chemical reaction is a system wherein one or more materials, additionally referred to as reactants, are converted into one or extra extraordinary materials referred to as merchandise. materials are either chemical elements or compounds. Chemical reactions rearrange the constituent atoms of reactants to produce extraordinary materials as merchandise.
The houses of the product vary from the ones of the starting cloth. A chemical reaction isn't the same as a bodily change that entails a exchange of country, along with ice melting to water or water evaporating to steam. while a physical trade takes place, the bodily homes of count change, but its chemical identification stays the identical.
Learn more about The reaction here:- https://brainly.com/question/11231920
#SPJ4
Passage 1
Passage 2
Dorothy Parker (1893-1967) was an American short-story
writer, poet, and critic best known for her satiric wit
This excerpt is from her essay titled Henry' in her book
Men Im Not Married To.
from "Henry"
by Dorothy Parker
You would really be surprised at the number of
things that Henry knows just a shade more
about than anybody else does. Naturally he
can't help realizing this about himself, but you
mustn't think for a minute that he has let it
spoil him. On the contrary... [w]hen it comes
to giving his time and his energy there is
nobody who could not admit that Henry is
Passage 1
Passage 2
Passage 1 Passage 2
Drag ONE sentence to EACH box to compare and
contr themes in Passage 1 and Passage 2.
Both
generous. To a fault, I have even heard people
go so far as to say.
... And that is the way Henry is about
everything. He will stroll over to a tennis court,
and stand on the side lines, at what I am sure
must be great personal inconvenience, calling
words of advice and suggestion for sets at a
stretch. I have even known him to follow his
friends all the way around a golf course,
offering constructive criticism on their form as
he goes. I tell you, in this day and generation,
you don't find many people who will go as far
out of their way for their friends as Henry does.
And I am far from being the only one who says
so, too.
strong friendships are based on emotion rather than
intellect.
Friends who remain detached from others may risk
friendships that expose their faults.
Friends who flaunt their perceived superiority may
risk losing friendships.
Most friendships are complicated because people are
imperfect.

Answers
if two successive terms of the fibonacci sequence are both odd, is the next term is even or odd? why?
Answers
Answer:
Even because you always add the previous two terms in the sequence. Since they are both odd, the next term will be even. (odd+odd = even)
Explanation:
8. What does the term 'sustainable mean? *

Answers
Answer:
something that can be maintained over a period of time
Answer:
a balance between meeting today's needs.......
Explanation:
the mass in g of 3 moles Al .
Answers
Answer:
81 gm
Explanation:
The mass of 1 mole is 27 g
So, Mass of 3 moles of Aluminium would be 3 × 27 = 81 gm..Thus mass of 3 Moles of Aluminium is 81 gm.
How do I do these problems? To give all possible ml values for the orbitals that have each of the following values

Answers
When n=2 this means that the electron is located on the second energy level. We then calculate the momemtum quantum number which is n-1. In this case the momentum quantum number is 1 (l=1). The magnetic quantum number ml is given by -l to +l. Therefore when n=2, the ml= -1,0,+1
When n=6 and l=5, ml would have values -5 to +5.
ml values are: -5,-4,-3,-2,-1,0,1,2,3,4,5
– Describe the subatomic particles that make up a neutral atom of carbon-12.
Include in the description:
– the number of each particle
– the location of each particle
– Which of the particles are responsible for the atomic number of carbon-12?
– Justify your response.
Answers
Answer:
Maybe this is what your looking for
Isotopes are various forms of an element that have the same number of protons but a different number of neutrons. Some elements, such as carbon, potassium, and uranium, have multiple naturally-occurring isotopes. Isotopes are defined first by their element and then by the sum of the protons and neutrons present.
Carbon-12 (or 12C) contains six protons, six neutrons, and six electrons; therefore, it has a mass number of 12 amu (six protons and six neutrons).
Carbon-14 (or 14C) contains six protons, eight neutrons, and six electrons; its atomic mass is 14 amu (six protons and eight neutrons).
While the mass of individual isotopes is different, their physical and chemical properties remain mostly unchanged.
Isotopes do differ in their stability. Carbon-12 (12C) is the most abundant of the carbon isotopes, accounting for 98.89% of carbon on Earth. Carbon-14 (14C) is unstable and only occurs in trace amounts. Unstable isotopes most commonly emit alpha particles (He2+) and electrons. Neutrons, protons, and positrons can also be emitted and electrons can be captured to attain a more stable atomic configuration (lower level of potential energy ) through a process called radioactive decay. The new atoms created may be in a high energy state and emit gamma rays which lowers the energy but alone does not change the atom into another isotope. These atoms are called radioactive isotopes or radioisotopes.
Explanation:
Isotopes are various forms of an element which have the same number of protons but a different number of neutrons, like carbon, potassium, and uranium, have multiple naturally-occurring isotopes.
what is carbon isotopes ?Carbon-12 shows six protons, six neutrons, and six electrons; it has a mass number of 12 amu (six protons and six neutrons).
Carbon-14 (or 14C) have six protons, eight neutrons, and six electrons; it has the atomic mass is 14 amu (six protons and eight neutrons).
The mass of individual isotopes is different where as physical and chemical properties remain mostly unchanged. Carbon-12 (12C) is the most abundant of the carbon isotopes which accounting for 98.89% of carbon on Earth.
Carbon-14 (14C) is unstable and it only occurs in trace amounts. Unstable isotopes emit alpha particles (He2+) and electrons. Neutrons, protons, and positrons.
For more details isotope, visit
https://brainly.com/question/11394246
#SPJ2
What units are carbon emissions measured in?
Answers
Answer:
GHG emissions are often measured in carbon dioxide (CO2) equivalent. To convert emissions of a gas into CO2 equivalent, its emissions are multiplied by the gas's Global Warming Potential (GWP).
A sample of trifluoroacetic acid, C2HF3O2, contains 77.7 g of fluorine. Calculate the mass of the trifluoroacetic acid sample.
Answers
The mass of trifluoroacetic acid, C₂HF₃O₂ which contains 77.7 g of fluorine is 155.4 grams
How do I determine the mass of trifluoroacetic acid, C₂HF₃O₂?We'll begin by obtaining the mass of C₂HF₃O₂ in one mole. Details below:
1 mole of C₂HF₃O₂ = (12 × 2) + 1 + (19 × 3) + (16 × 2) = 114 gMass of fluorine, F in 1 mole of C₂HF₃O₂ = 3F = 3 × 19 = 57 gThus, we can say:
57 grams of fluorine, F are present in 114 grams of C₂HF₃O₂
With the above information, we can obtain the mass of C₂HF₃O₂ that contains 77.7 g of fluorine. Details below:
57 grams of fluorine, F are present in 114 grams of C₂HF₃O₂
Therefore,
77.7 grams of fluorine, F will be present in = (77.7 grams × 114 grams) / 57 grams = 155.4 grams of C₂HF₃O₂
Thus, from the above calculation, we can conclude that the mass of trifluoroacetic acid, C₂HF₃O₂ is 155.4 grams
Learn more about mass composition:
brainly.com/question/9274824
#SPJ1
871g of sodium chloride is how many moles
Answers
Answer:
14.9 mol
Explanation:
To find the number of moles in a given mass of a sample of sodium chloride (NaCl), we can multiply the number of grams in the sample by the molar mass of sodium chloride, which is 58.44 g/mol.
871 g × (1 mol / 58.44 g)
= 871/58.44 mol
≈ 14.9 mol
Note that we rounded to 3 significant figures in the final answer because that is how many significant figures were given in the mass measurement of the sodium chloride sample.
Consider the reaction of solid aluminum iodide and potassium metal to form solid potassium iodide and aluminum metal.
Write the balanced chemical equation for the reaction.
Answers
The balanced chemical equation is AlI₃(s) + 3 K(s) → 3 KI(s) + Al(s). The number of moles of aluminium will be 2.437moles.
What is chemical equation ?Chemical equations are symbols and chemical formulas that depict a chemical reaction symbolically.The reactive species and end product are described by the chemical equation. The mole ratio or molecular ratio of the constituent components or compounds in the reaction is revealed by the coefficient of the reacting species and the products that are produced.Reactants are the substance(s) in a chemical equation to the left of the arrow. A component that is present at the outset of a chemical reaction is known as a reactant. Products are the substance(s) to the right of the arrow. A substance that remains after a chemical reaction is complete is known as a product.To learn more about chemical equation refer :
https://brainly.com/question/26227625
#SPJ1
Which graph represents a function?
Answers
Answer:
I need to se the options
What kind of energy transfers between molecules of touching substances during conduction
Answers
Answer:
heat energy
Explanation:
Conduction is the transfer of heat energy from one substance to another or within a substance.
When all concentrations at equilibrium are known a numerical value for the constant can be calculated. Give the value for the Keq given the following concentrations:
[HF] = 0.030 M, [H3O+1] = 0.020 M, [F-1] = 0.020 M
HF + H2O ⇔ F-1 + H3O+1
[F-1][H3O+1] [
][ ]
Keq = ––––––––––– = ––––––––––––––––––––––––––––––––––––––––––––––––– =
[HF] [ ]
a. HF b. H2O C. F-1 d. H3O+1 e.0.020 M f. 0.030 M G. 0.013
Units are not usually used with equilibrium constants. This Keq can also be called a Ka since it is for an acid.
Answers
Answer:
Keq = 0.013
Explanation:
Keq is the ratio of the concentrations of the products to the concentration of the reactants.
For any reaction aA+bB->cC+dD (a,b,c,d are coefficients), \(K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}\).
Furthermore, pure solids and liquids are omitted from these Keq expressions; the only things included in Keq expressions are aqueous or gaseous substances.
Since the coefficients for all reactants and products in the given reaction is 1 and water is omitted because it is a pure liquid, \(K_{eq}=\frac{[F^-][H_3O^+]}{[HF]}\).
Plugging in the concentrations gives \(K_{eq}=\frac{0.020*0.020}{0.030}=\frac{0.020^2}{0.030}=\frac{0.00040}{0.030}=0.013\).
in an oxide of tungsten, the oxygen anions (o2-) are located halfway along all of the edges of the cubic unit cell, and the tungsten cations occupy positions at each corner of the unit cell. the density of the oxide is 6.013 g/cm3. the molar mass of w is 183.80 g/mol and the molar mass of o is 16.00 g/mol. (a) Provide the empirical formula of the oxide.
(b) Calculate the lattice parameter of the cubic unit cell.
Answers
The Edge length of unit cell is 4*10⁻⁸ cm.
What is tungsten?
Chemical element tungsten (W), often known as wolfram, is a Group 6 (VIb) refractory metal that is incredibly strong and is used to make lamp filaments and to boost the hardness and strength of steels.
What is cations ?
A positively charged cation is an atom or molecule with more protons than electrons. Eg: Na+ 3. An anion is an atom or molecule that is negatively charged, meaning it contains more electrons than protons.
O⁻²⇒ mid of all edges
no.of ion shared from 1 edge = 1/4
total edges = 12
total o⁻² ions = 12*1/4
total o⁻² ions = 3
tungsten caution ⇒ each corner
no.of ions shared from corner = 1/8
total corner = 8
total tungsten ion = 8*1/8
total tungsten ion = 1
empirical formula = wo₃
b) density = Z*M/Na * a³
Here, Z= 1( number of molecule in 1 Unit cell)
M= molar mass of WO₃= 231.84 gm / mole
Na = Avogadro number = 6.022*10²³
a= edge length
6.013= 1*231.84/ 6.022*10²³ *a³
a³= 231.84 * 10⁻²³ / 6.013 * 6.022
a³= 6.403 * 10⁻²³
a³= 6.403 * 10⁻²⁴
a= 4*10⁻⁸ cm
Therefore, the Edge length of unit cell is 4*10⁻⁸ cm.
Learn more about tungsten from the given link.
https://brainly.com/question/29624740
#SPJ4
I need help finding both conjugate acid and base

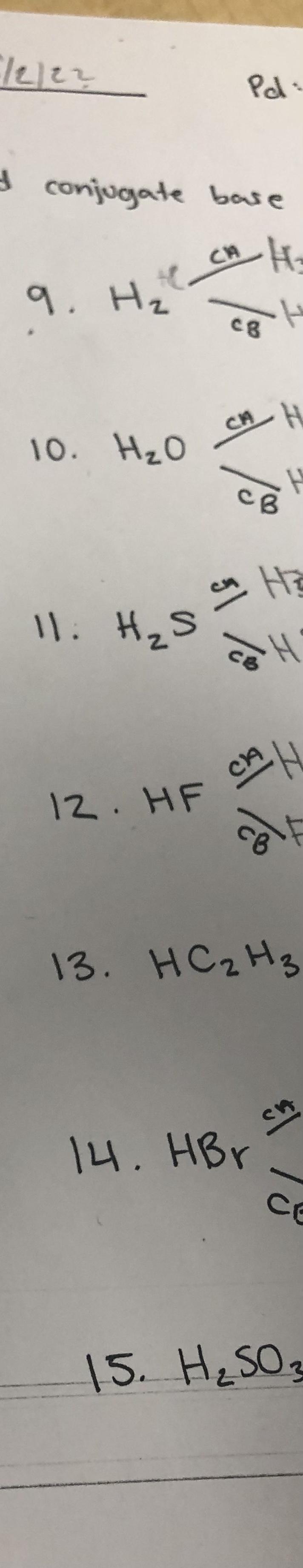
Answers
What is the density of an unknown compound in g/ml if 1.28 pounds of the compound has a volume of 4.50L
Answers
1.28 pounds * 453.59 grams/pound = 580.61 grams
Next, we can use the formula for density:
Density = Mass / Volume
Density = 580.61 grams / 4.50 L
Density = 128.91 g/L
Therefore, the density of the unknown compound is 128.91 g/L or 0.12891 g/mL (since there are 1000 mL in 1 L).
PLEASE HELP ON QUESTION

Answers
Answer:
unbalanced, direction of movement: left
Explanation:
because im smart
Answer:
unbalanced, direction of movement: to the left
Explanation:
Hope this helps :)