Answers
Correct option a) The candle wax turns liquid as it burns.
The wax close to flame burns and offers new materials like carbon dioxide, carbon soot, water vapour, warmth and light.When a candle burns, wax withinside the candle melts and is then vaporised as it's miles drawn up the wick.
Melting and vaporisation are bodily modifications. The wax vapours then burn on the wick to depart in the back of soot and water vapour, whilst emitting warmth and light. The burning of wax vapours is a chemical change. Thus, wax undergoes each bodily and chemical modifications while a candle burns.When a candle burns, each bodily & chemical modifications occur.
On heating, candle wax melts and shape liquid wax. It is a bodily change. Since it once more will become stable wax on cooling. This is a reversible change. When a candle is burnt in air, the mass of the product is expanded because of atmospheric oxygen which display a that it's miles a chemical change.
To learn more about reversible change here
https://brainly.com/question/14831707
#SPJ1
Related Questions
After some salt was added to it, a 45.4 g solution in a coffee-cup calorimeter increased in temperature from 23.0 oC to 31.5 oC.
The specific heat constant
(c) for the solution is 1 cal/g oC. The q of the reaction is ________ cal
Answers
The heat absorbed by the solution is 386.9 cal.
First, we need to calculate the heat absorbed by the solution using the formula:
q = m × c × ΔT
Where q is the heat absorbed, m is the mass of the solution, c is the specific heat constant, and ΔT is the change in temperature.
m = 45.4 g
c = 1 cal/g oC
ΔT = 31.5 oC - 23.0 oC = 8.5 oC
Substituting the values in the formula, we get:
q = 45.4 g × 1 cal/g oC × 8.5 oC
q = 386.9 cal
Therefore, the heat absorbed by the solution is 386.9 cal.
Learn more about heat absorbed, here:
https://brainly.com/question/12473602
#SPJ1
100-99+98-97+...+4-3+2-1
Answers
Answer:
The answer is 4
Explanation:
=1+98−97+4−3+2−1
=99−97+4−3+2−1
=2+4−3+2−1
=6−3+2−1
=3+2−1
=5−1
=4
Fe(NO3)3
Percent composition
Answers
Answer:
Fe: 23.1%
N: 17.4%
O: 59.5%
Explanation:
You take the grams that each weigh on the periodic table and add it all up. Afterward you create fractions of each over the whole. (Example: Fe/Fe+N+O) then you divide and turn that decimal into a percentage.
What would the approximate age of an
igneous rock that contains only 1/4 of its
original carbon-14 (half-life of carbon is
5700 years)
Answers
Explanation:
Carbon-14 has a half life of 5730 years, meaning that 5730 years after an organism dies, half of its carbon-14 atoms have decayed to nitrogen atoms. Similarly, 11460 years after an organism dies, only one quarter of its original carbon-14 atoms are still around.
When 1.00-gram sample of baking soda is heated, a gas is formed. The solid that remains weighs less than 1.00 g and does not have the same properties as the original sample. Is baking soda an element or compound?
Answers
Answer:
Compound
Hope it helps!
Using your knowledge of boiling points and intermolecular forces, why do you think rubbing alcohol dries so quickly from your skin?
Answers
Answer:
Due to its weak intermolecular forces which make it volatile.
Explanation:
Hello,
In this case, antiseptic alcohol which chemically named as ethanol has a low boiling point (78.37 °C) and weak intermolecular forces of dipole-dipole kind, which make it volatile, it means it tends to rapidly vaporize even when it is not gently heated, therefore, even a weak force such as rubbing it, is enough to vaporize it out from the skin so it quickly dries.
Best regards.
Due to low boiling point and weak intermolecular force alcohol is volatile, which makes it to dry quickly from the skin.
Volatility of alcohol• The substances with strong intermolecular forces exhibit lower vapor pressures and are less volatile, on the other hand, the substances with weak intermolecular forces exhibit higher vapor pressures and are more volatile.
• Ethanol, that is, the antiseptic alcohol exhibits a low boiling point of 78.37 degree C and weak intermolecular forces of dipole-dipole kind.
• The characteristics of low boiling point and weak intermolecular forces makes the alcohol volatile.
• It shows that even when it is gently rubbed onto the skin, it vaporizes.
Thus, due to the properties of alcohol, even a weak force like rubbing is adequate to vaporize it out from the skin, and therefore, it dries briskly.
Find out more information about volatility of alcohol here:
https://brainly.com/question/17144805
The combustion of octane, C8H18, proceeds according to the reaction shown.
2C8H18(l)+25O2(g)⟶16CO2(g)+18H2O(l)
If 354 mol of octane combusts, what volume of carbon dioxide is produced at 15.0 ∘C
and 0.995 atm?
Answers
The concept ideal gas equation is used here to determine the volume of the carbondioxide. Combustion reactions are generally highly exothermic reactions. The volume of CO₂ is
A combustion is a chemical reaction in which a fuel undergoes oxidation as a result of the reaction with an oxidizing agent which causes the release of energy in the form of heat.
15.0 °C = 288 K
The ideal gas equation is:
PV = nRT
V = nRT / P
V = 354 × 0.0821 × 288 / 0.995 = 8412.3 L
To know more about combustion, visit;
https://brainly.com/question/14335621
#SPJ1
4.) What % of the original atoms remained after 8 trials?
Answers
The remaining percentage of the original radioactive atoms is 321100=3. 125%.
What percentage of radioactive substance remains after ten half-lives?We have 12.5 percent left after another six hours. Only 0.1% of the radioactivity is left after ten half-lives. Three different half-lives are available.More than 99 percent of an atom's volume is actually empty space when protons, neutrons, and electrons are taken into account.According to current estimates, hydrogen makes up 90% of all atoms in the universe and is crucial to the existence of the material world.Therefore, 50% of the original parent nuclei are still there after one half-life; 25% are still present after two half-lives; and so on. A radioactive source's half-life and the initial quantity of radioactive atoms present affect the radiation's intensity.To learn more about percentage refer to:
https://brainly.com/question/24304697
#SPJ1
Balance the following equation. ___MgCI2+ ___ AgNO3-->___Mg(NO3)2
Answers
Answer:
MgCI2+ ___ AgNO3-->___Mg(NO3)2
i think u put 2 before NO3
Explanation:
Which molecule is butane?
H H H H
A. H-C-C-C-C-H
||||
H H H H
B.
C.
H3C
C=C
H
CH3
H
H
|
D. H-C=C-C-C-H
H H
H
|
Answers
Answer: A
Explanation:
The -ane suffix implies that the compound has only single bonds for carbon-carbon bonds. The but- prefix implies that the compound consists of four carbons. Since 4 bonds are required for each carbon, there will be a total of 10 hydrogen atoms: 3 on each carbon at the end of the chain and 2 for each carbon in the middle of the chain. Thus, butane is A.
A helium balloon with an internal pressure of 1.00 atm and a volume of 4.50 L at 20.00 C is released. What volume will the balloon occupy at an altitude where the pressure is 0.600 atm and the temperature is -20.00 C?
Answers
The volume the balloon will occupy at an altitude where the pressure is 0.600 atm and the temperature is -20.00 C is 6.15 L.
The ideal gas law can be used to solve the given problem, which is as follows:P1V1/T1 = P2V2/T2 Where:P1 is the initial pressure (1.00 atm)P2 is the final pressure (0.600 atm)V1 is the initial volume (4.50 L)V2 is the final volume (what we want to find)T1 is the initial temperature (20.00 C = 293.15 K)T2 is the final temperature (-20.00 C = 253.15 K)The problem does not provide the mass or number of moles of helium gas, but these quantities are not required to solve the problem as they cancel out in the calculation.The problem also does not mention any change in altitude, so we can assume that the balloon rises to an altitude where the pressure and temperature are lower.We can rearrange the equation above to solve for V2, which gives:V2 = (P1V1T2)/(P2T1) Substituting the given values gives:V2 = (1.00 atm × 4.50 L × 253.15 K)/(0.600 atm × 293.15 K)V2 = 6.15 L.
for such more questions on volume
https://brainly.com/question/29796637
#SPJ8
What is the unit of force
Answers
Answer:
the unit of force is newton represented with symbol "N"
Name the subatomic parts of the atom, their charges and where they are located.
Answers
Answer:
protons: positively charged, located in the nucleus
electrons: negatively charged, located outside the nucleus
neutrons: no charge, located in the nucleus
What branch of chemistry studies the flow of electrons?
A. Inorganic chemistry
B. Electrochemistry
C. Quantum chemistry
D. Organic chemistry
Answers
Answer:
Your answer is B, Electrochemistry!
Explanation:
This is the part of chemistry that studies the chemical process in which electrons flow. This flow is called electricity. Electricity is generated by the flow of electrons, from one element to another element. This reaction is called oxidation reduction.
Answer: B
Explanation:
Rick is creating a love potion for Morty. To make the potion, Rick's needs 51 mL of a mixture solution where 40% is carbonated water. After checking around his shop, Rick finds two solutions he could use. The first solution he found is 65% green tea, 15% carbonated water, and 20% whole milk. The second solution is 17% orange juice, 38% lemonade, and 45% carbonated water. How much of the first solution and second solution does Rick need to mix together to create the love potion? Round your final answers to one decimal place. You may solve this problem using any method we have learned in the class.
Answers
Answer:
The amount of the first solution rick needs to mix together to create the love portion is 8.5 mL
Explanation:
So as to make the love potion, we have;
The percentage of carbonated water in the love portion = 40%
The percentage of green tea in the first solution = 65%
The percentage of carbonated water in the first solution = 15%
The percentage of whole milk in the first solution = 20%
The percentage of orange juice in the second solution = 17%
The percentage of lemonade in the second solution = 38%
The percentage of carbonated water in the second solution = 45%
Let 'x' represent the volume in mL of the first solution added to make the love portion, and let 'y' be the volume in mL of the second solution added to make the love portion, we have;
x + y = 51...(1)
0.15·x + 0.45·y = 0.40×51 = 20.4
0.15·x + 0.45·y = 20.4...(2)
Solving the system of simultaneous equation by making 'y' the subject of each of the equation gives;
For equation (1)
y = 51 - x
For equation (2)
y = 20.4/0.45 - (0.15/0.45)·x = 136 - 3·x
y = 136/3 - (1/3)·x
Equating the two equations of 'y', gives;
51 - x = 136/3 - (1/3)·x
51 - 136/3 = x - (1/3)·x
17/3 = (2/3)·x
(2/3)·x = 17/3
x = (3/2) × (17/3) = 17/2 = 8.5
x = 8.5
y = 51 - x = 42.5
y = 42.5
Therefore, the amount of the first solution rick needs to mix together to create the love portion, x = 8.5 mL
Will you answer this for me ?

Answers
Answer:
Explanation:
balance
2 C2H6 + 7 O2 --> 4 CO2 + 6 H2O
given 360 g H20 (g)
required =586.67 g CO2
360 g H20 x (1mole/18 g H20) X (4 mole CO2/6 moles H20) X (44g CO2/1mole) =586.67 g CO2
Cryolite, Na3AlF6(s),
an ore used in the production of aluminum, can be synthesized using aluminum oxide.
equation:
Al2O3(s)+6NaOH(l)+12HF(g)⟶2Na3AlF6+9H2O(g)
If 10.3 kg of Al2O3(s),
55.4 kg of NaOH(l),
and 55.4 kg of HF(g)
react completely, how many kilograms of cryolite will be produced?
mass of cryolite produced:
Answers
The mass (in kilograms) of Cryolite, Na₃AlF₆ produced, given that 10.3 Kg of Al₂O₃, 55.4 Kg of NaOH, and 55.4 Kg of HF react completely is 42.4 Kg
How do i determine the mass of Na₃AlF₆ produced?The mass of Na₃AlF₆ produced from the reaction can be obtained as follow:
Al₂O₃(s) + 6NaOH(l) + 12HF(g) ⟶ 2Na₃AlF₆ + 9H₂O(g
Molar mass of Al₂O₃ = 102 g/molMass of Al₂O₃ from the balanced equation = 1 × 102 = 102 g = 102 / 1000 = 0.102 KgMolar mass of Na₃AlF₆ = 210 g/molMass of Fe from the balanced equation = 2 × 210 = 420 g = 420 / 1000 = 0.420 KgFrom the balanced equation above,
0.102 Kg of Al₂O₃ reacted to produce 0.420 Kg of Na₃AlF₆
Therefore,
10.3 Kg of Al₂O₃ will react to produce = (10.3 × 0.420) / 0.102 = 42.4 Kg of Na₃AlF₆
Thus, from the above calculation, it is evident that the mass of Na₃AlF₆ produced is 42.4 Kg
Learn more about mass produced:
https://brainly.com/question/9526265
#SPJ1
A sample of a certain lead compound contains 12.92 g of lead for 2 g of oxygen. A second sample has mass of 34.27 g and contains 14.39 g of oxygen. Are the two compound the same
Answers
The two chemical compounds are not the same, because their ratio is not equal. In both samples the composition of lead and oxygen is different.
What is a chemical compound?A chemical compound is a substance made of numerous similar molecules (or molecular entities) joined by chemical bonds and comprising atoms from various chemical elements. Therefore, a molecule made up of only one type of atom is not a compound. Chemical reactions, which may entail interactions with other molecules, can change a compound into a distinct substance. Atomic bonds may be broken or new ones created during this process.
What are the calculations?sample 1 = mass of lead / mass of oxygen = 12.92g/2g = 6.46 .
sample 2 = mass of lead/ mass of oxygen = 34.27 - 14.39/14.39 = 1.38 .
so, the ratios are not the same.
Hence, the two chemical compounds are not the same, because their ratio is not equal. In both samples the composition of lead and oxygen is different.
To know more about Chemical compounds, check out:
https://brainly.com/question/26487468
#SPJ1
What law allows calories to be determined by heat (energy) transfer from one substance to another, but it is never destroyed?
Answers
Answer:
matter i think
Explanation:
matter i am pretty sure
The law that allows calories to be determined by heat (energy) transfer from one substance to another, but it is never destroyed is first law of thermodynamics.
What is first law of thermodynamics?Energy cannot be generated or destroyed, only changed in form, according to the basic law of thermodynamics.
Energy transfer occurs when mass crosses the control boundary, external work is performed, or heat is transferred across the boundary in any system. These cause the stored energy in the control volume to shift.Hence first law of thermodynamics justify the given statement.
To know more about thermodynamics, visit the below link:
https://brainly.com/question/26035962
#SPJ2
A boy sets up a picnic on the beach next to a sand dune. How are sand dunes formed?
A. erosion and deposition
B. weathering and erosion
C. tectonic plates
D. precipitation
Answers
Answer:
B. Weathering and Erosion
Explanation:
I majored in Chemistry
Answer:
b
Explanation:
Calculate the heat change (ΔΗ°rxn) for the slow reaction of zinc with water
Zn(s)+2H2O(l) ---> Zn^2+ (aq) +H2(g)
ΔΗ°rxn = kJ
Answers
The heat change or enthalpy change, ΔH°rxn, for the slow reaction of zinc with water is +417.7 kJ/mol.
The heat change or enthalpy change, ΔH°rxn, for the reaction of zinc (Zn) with water (H₂O) can be calculated using the standard enthalpies of formation for each species involved in the reaction.
Balanced chemical equation for the reaction is;
Zn(s) + 2H₂O(l) → Zn²⁺(aq) + H₂(g)
The standard enthalpy change for the reaction, ΔH°rxn, can be calculated as the sum of the standard enthalpies of formation of the products minus the sum of the standard enthalpies of formation of the reactants, each multiplied by their respective stoichiometric coefficients;
ΔH°rxn = Σ(nΔH°f, products) - Σ(mΔH°f, reactants)
where n and m are the stoichiometric coefficients of the products and reactants, respectively, and ΔH°f is the standard enthalpy of formation.
Assuming standard conditions (25°C and 1 atm), the standard enthalpies of formation for Zn²⁺(aq) and H₂(g) are typically tabulated values. Let's assume their values to be ΔH°f(Zn²⁺(aq)) = -153.9 kJ/mol and ΔH°f(H₂(g)) = 0 kJ/mol, respectively.
The standard enthalpy of formation of water (H₂O) is -285.8 kJ/mol.
Put the values into the equation, we get;
ΔH°rxn = [ΔH°f(Zn²⁺(aq)) + ΔH°f(H₂(g)] - [ΔH°f(Zn(s)) + 2ΔH°f(H₂O(l))]
ΔH°rxn = [-153.9 + 0] - [0 + 2(-285.8)]
ΔH°rxn = -153.9 + 571.6
ΔH°rxn = 417.7 kJ/mol
To know more about heat change here
https://brainly.com/question/18912282
#SPJ1
PLEAS HELP I HAVE LIMITED TIME!!
Which compound is the limiting reagent?
Select one:
O2
O H20
H2
Cannot be determined.
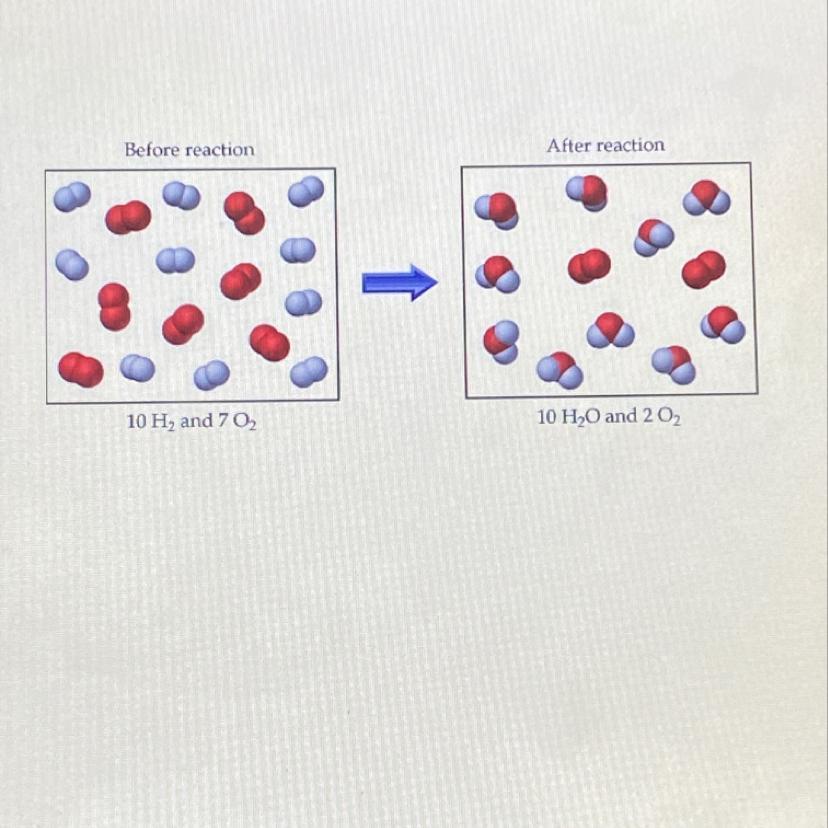
Answers
Answer:
H2 is the limiting reactant.
Explanation:
From the diagram above:
H2 => White ball
O2 => Red ball
Before the reaction
H2 => White ball => 10
O2 => Red ball => 7
After the reaction
H2O => White and red ball => 10
O2 => 2
From the simple illustration above, we can see that all the H2 were used up in the reaction but there are left over of O2.
This simply means that H2 is the limiting reactant as all of it is used up in the reaction while O2 is the excess reactant as there are leftover.
Can H2 be broken down? (Not H)
Answers
Hello, this is Bing. I can help you with your question. Based on the information I found on the web, **H2** can be broken down into its two atoms of hydrogen (H) by supplying enough energy to overcome the bond that holds them together⁴. This process is called **dissociation** and requires an energy equal to or greater than the **dissociation energy** of H2, which is about 436 kJ/mol⁴.
One way to break down H2 is by using **electricity** to split water (H2O) into hydrogen (H2) and oxygen (O2) through a process called **electrolysis**¹. In this process, water is decomposed into its elements by passing an electric current through it. The electric current is provided by a battery or another source of electricity and the water needs to have an **electrolyte**, such as salt or acid, added to it to make it conductive¹. Two electrodes, usually made of metal or other conductive material, are inserted into the water and connected to the battery. The electrode connected to the positive terminal of the battery is called the **anode** and the one connected to the negative terminal is called the **cathode**¹. When the electric current flows through the water, hydrogen gas bubbles form at the cathode and oxygen gas bubbles form at the anode¹. The overall chemical reaction for electrolysis of water is:
2 H2O → 2 H2 + O2
Another way to break down H2 is by using **heat** to cause a reaction between hydrogen and oxygen that produces water and releases a large amount of energy. This reaction is called **combustion** or **oxidation** and can be ignited by a spark or a flame³. The reaction is very fast and explosive and can be dangerous if not controlled. The overall chemical reaction for combustion of hydrogen is:
2 H2 + O2 → 2 H2O
I hope this helps you understand how H2 can be broken down and what methods are used to do so.
how many moles of CaCo3 are there in an antacid tablet containing 0.512g CaCo3
Answers
The number of mole of CaCO₃ in antacid tablet containing 0.512 g of CaCO₃ is 5.12×10⁻³ mole
Description of moleThe mole of a substance is related to it's mass and molar mass according to the following equation:
Mole = mass / molar mass
How to determine the mole of CaCO₃From the question given above, the following data were obtained:
Mass of CaCO₃ = 0.512 gMolar mass of CaCO₃ = 40 + 12 + (3 × 16) = 40 + 12 + 48 = 100 g/mol Mole of CaCO₃ =?The number of mole in 0.512 g of CaCO₃ can be obtained as follow:
Mole = mass / molar mass
Mole of CaCO₃ = 0.512 / 100
Mole of CaCO₃ = 5.12×10⁻³ mole
Thus, 5.12×10⁻³ mole of CaCO₃ is present in the antacid tablet
Learn more about mole:
https://brainly.com/question/13314627
#SPJ1
You see the color orange in the neon sign above because electrified neon atoms produce a single orange colored wavelength of light.
Answers
Answer: As a result, each excited electron in an atom emits a photon of a specific wavelength. To put it another way, each excited noble gas emits a distinct hue of light. This is a reddish-orange neon light.
which stage takes place before the one in the diagram above
Answers
The stage that takes place before the one in the diagram above is metaphase II
What is metaphase II?Metaphase II is a stage in meiosis II, which is the second round of cell division that occurs during meiosis. Meiosis is a process of cell division that produces haploid cells (cells with half the number of chromosomes as the parent cell) from diploid cells (cells with the full number of chromosomes). Meiosis II begins after the completion of meiosis I, which produces two haploid cells from one diploid cell.
During metaphase II, the replicated chromosomes (each consisting of two sister chromatids) line up at the metaphase plate, which is an imaginary line that divides the cell into two halves.
Learn more about metaphase:https://brainly.com/question/9360168
#SPJ1

What are some potential real-world applications for renewable energy sources such as solar power and wind power?
Answers
The some of the potential in the real world applications for the renewable energy sources such as the solar power and the wind power are electricity generation, the water heating and cooling, and the transportation.
Renewable energy defined as the energy produced from the sources like the sun and the wind energy which are the naturally replenished and which do not run out.
The Renewable energy which can be used for the electricity generation, and the water heating and the cooling, and the transportation. The most sustainable sources of the energy are the renewable bioenergy. The Renewable sources of the, like the wind and the solar, it will emit the little to no the greenhouse gases.
To learn more about renewable energy here
https://brainly.com/question/18004988
#SPJ1
I need help please on chemistry

Answers
The basic unit of structure and function of living things is the .
nucleus
cell
tissue
membrane
Answers
Answer &
Explanation:
eukaryotic: Having complex cells in which the genetic material is contained within membrane-bound nuclei. cell: The basic unit of a living organism, consisting of a quantity of protoplasm surrounded by a cell membrane, which is able to synthesize proteins and replicate itself.
therefore it is cell
Write the chemical symbol for neon-21.
Answers
Answer:
(~21~ Ne)Neon | Ne-pubchem