what causes the earth to have layers?
Answers
Related Questions
you want 200 mL of a 0.1 g/mL solution of sugar. how do you prepare the solution?
Answers
what species are produced at the positive and negative electrodes during the electrolysis of molten sodium chloride?
Answers
The species are produced at the positive and negative electrodes during the electrolysis of molten sodium chloride is at the positive and the species is Cl₂(g) and at the end of negative is Na(l).
The net effect of the passing the electric current to the molten salt in this cell that is to decompose the sodium chloride into the its elements, the sodium metal and the chlorine gas. The anode is called as the positive end and the cathode is called as the negative end.
During the process of the electrolysis the chlorine gas are produce at the positive end that is anode. The sodium metal is produce at the negative end that is the catode.
To learn more about electrolysis here
https://brainly.com/question/9977624
#SPJ4
Aluminum nitrite and ammonium chloride react to form aluminum chloride, nitrogen, and water. What mass of each substance is present after 63.8 g aluminum nitrite and 52.5 g ammonium chloride react completely?
Answers
Taking into account definition of reaction stoichiometry, after 63.8 g aluminum nitrite and 52.5 g ammonium chloride react completely, there are present a mass of 43.66 grams of AlCl₃, 27.50 grams of N₂, 35.36 grams of H₂O and 9.78 grams of Al(NO₂)₃.
Reaction stoichiometryIn first place, the balanced reaction is:
Al(NO₂)₃(aq) + 3 NH₄Cl(aq) → AlCl₃(aq) + 3 N₂(g) + 6 H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Al(NO₂)₃: 1 moleNH₄Cl: 3 molesAlCl₃: 1 molesN₂: 3 molesH₂O: 6 molesThe molar mass of the compounds is:
Al(NO₂)₃: 165 g/moleNH₄Cl: 53.45 g/moleAlCl₃: 133.35 g/moleN₂: 28 g/moleH₂O: 18 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Al(NO₂)₃: 1 mole ×165 g/mole= 165 gramsNH₄Cl: 3 moles ×53.45 g/mole= 160.35 gramsAlCl₃: 1 mole ×133.35 g/mole= 133.35 gramsN₂: 3 moles ×28 g/mole= 84 gramsH₂O: 6 moles ×18 g/mole= 108 gramsLimiting reagentThe limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
Limiting reagent in this caseTo determine the limiting reagent, it is possible to use a simple rule of three as follows: if by stoichiometry 165 grams of Al(NO₂)₃ reacts with 160.35 grams of NH₄Cl, 63.8 grams of Al(NO₂)₃ reacts with how much mass of NH₄Cl?
mass of NH₄Cl= (63.8 grams of Al(NO₂)₃× 160.35 grams of NH₄Cl)÷165 grams of Al(NO₂)₃
mass of NH₄Cl= 62.002 grams
But 62.002 grams of NH₄Cl are not available, 52.5 grams are available. Since you have less mass than you need to react with 63.8 grams of Al(NO₂)₃, NH₄Cl will be the limiting reagent.
Mass of each product formedConsidering the limiting reagent, the following rule of three can be applied: if by reaction stoichiometry 160.35 grams of NH₄Cl form 133.35 grams of AlCl₃, 52.5 grams of NH₄Cl form how much mass of AlCl₃?
mass of AlCl₃= (52.5 grams of NH₄Cl× 133.35 grams of AlCl₃)÷ 160.35 grams of NH₄Cl
mass of AlCl₃= 43.66 grams
The following rule of three can be applied: if by reaction stoichiometry 160.35 grams of NH₄Cl form 84 grams of N₂, 52.5 grams of NH₄Cl form how much mass of N₂?
mass of N₂= (52.5 grams of NH₄Cl× 84 grams of N₂)÷ 160.35 grams of NH₄Cl
mass of N₂= 27.50 grams
The following rule of three can be applied: if by reaction stoichiometry 160.35 grams of NH₄Cl form 108 grams of H₂O, 52.5 grams of NH₄Cl form how much mass of H₂O?
mass of H₂O= (52.5 grams of NH₄Cl× 108 grams of H₂O)÷ 160.35 grams of NH₄Cl
mass of H₂O= 35.36 grams
Mass of Al(NO₂)₃ in excessThe following rule of three can be applied: if by stoichiometry 160.35 grams of NH₄Cl reacts with 165 grams of Al(NO₂)₃, 52.5 grams of NH₄Cl reacts with how much mass of Al(NO₂)₃?
mass of Al(NO₂)₃= (52.5 grams of NH₄Cl× 165 grams of Al(NO₂)₃)÷160.35 grams of NH₄Cl
mass of Al(NO₂)₃= 54.02 grams
If 63.8 grams of the compound are present, then the excess mass can be calculated as:
excess mass= 63.8 grams -54.02 grams
excess mass= 9.78 grams
Learn more about reaction stoichiometry:
https://brainly.com/question/14799692
#SPJ1
What is the volume of 2.5 moles of a gas at 0.6 atm and 17 °C? (R = 0.082 L atm/mol K)
identify variables:
Equation used:
Substitution into equation:
Answer (include units):
Answers
To Find:
Volume of gasFormula used:
PV = nRT [ideal gas equation]0°C = 273 K
So, 17°C = 290 K
Let the volume of gas be V
Using the formula, we get
0.6 x V = 2.5x0.082x290
V = 2.5x0.82x290/6
V = 99.08 Litres
So, volume of gas will be 99 litres approx.
If 0.484J of heat is added to 0.1372g of water, how much will the temperature increase?
0.263 °C
0.927 °c
0.843 °C
0.460 °C
Answers
Answer:
ΔT = 0.843 °C
Explanation:
Given data:
Heat added = 0.484 J
Mass of water = 0.1372 g
Temperature increase = ΔT = ?
Solution:
Formula:
Q = m.c. ΔT
Q = amount of heat absorbed or released
m = mass of given substance
c = specific heat capacity of substance
ΔT = change in temperature
Specific heat capacity of water is 4.184 j/g.°C
Now we will put the values in formula.
0.484 j = 0.1372 g × 4.184 j/g.°C× ΔT
0.484 j = 0.574j/°C× ΔT
ΔT = 0.484 j / 0.574j/°C
ΔT = 0.843 °C
If you spilled nails in a sand box what separation tool/method could you use to clean out the sandbox so it would be safe for children to play with it's:filter,screen,magnet,Evaporation
Answers
Answer: Magnet
Explanation:Using a magnet is the best separating technique to be deployed in this case. The nails are easily picked out by just holding a magnet over the sandbox.
Determine the [OH−] , Ph, and POH of a solution with a [H+] of 0. 00017 m at 25 °C.
Answers
The answer is \(\underline{12.954}\end{aligned}$$\)
\(\begin{aligned}&\mathrm{pH}=\underline{1.046} \\&{\left[\mathrm{OH}^{-}\right]=\underline{1.11 \times 10^{-13} \mathrm{M}}} \\&\mathrm{pOH}=\underline{12.954}\end{aligned}\)
Given:
\(\left[\mathrm{H}^{+}\right]=0.090 \mathrm{M}=9 \times 10^{-2} \mathrm{M} ; \mathrm{T}\\=25^{\circ} \mathrm{C}$$\mathrm{As}, \mathrm{pH}=-\log \left[\mathrm{H}^{+}\right]$\Rightarrow \mathbf{p H}=-\log \left(9 \times 10^{-2}\right)=\underline{1.046}$$\)
The equation for the self-ionization constant of water is
\($$\mathrm{Kw}=\left[\mathrm{H}^{+}\right]\left[\mathrm{OH}^{-}\right]$$and, $\mathrm{pKw}=\mathrm{pH}+\mathrm{pOH}$\)
Since at room temperature,
\($25^{\circ} \mathrm{C}: \mathrm{Kw}=1.0 \times 10^{-14}, \mathrm{pKw}=14$\)
\(\therefore \mathrm{Kw}=\left[\mathrm{H}^{+}\right]\left[\mathrm{OH}^{-}\right]=1.0 \times 10^{-14}$$\\\Rightarrow\left[\mathrm{OH}^{-}\right]=\left(1.0 \times 10^{-14}\right) \div\left[\mathrm{H}^{+}\right]\\=\left(1.0 \times 10^{-14}\right) \div\left[9 \times 10^{-2}\right]\\=0.111 \times 10^{-12}=1.11$$\times 10^{-13} \mathrm{M}$\)
And
\($$\begin{aligned}&\mathrm{pH}+\mathrm{pOH}=\mathrm{pKw}=14 \\&\Rightarrow \mathrm{pOH}=14-\mathrm{pH}=14-1.046=\underline{12.954}\end{aligned}$$\)
What is Poh and PH linked formula?
Take the negative log of the hydronium ion concentration and use that value to compute pH. Simply subtraction the pH from 14 yields the pOH value. The negative log of the concentration of hydroxide ions should be used to get the pOH. Simply subtraction 14 from pOH yields the pH.So the more about Poh and PH linked formula.
https://brainly.com/question/16867635
#SPJ4
What are the parts of an atom

Answers
Answer:
Electrons,Protons, and Neutrons
i thought everyone knew that
Can you help plzzz thank you
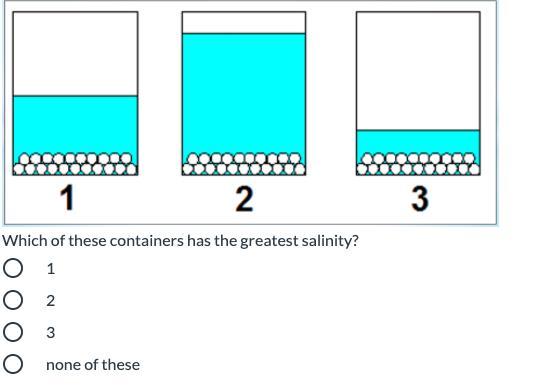
Answers
Answer:
It would be Container 3
Explanation:
Each of the containers has the same amount of salt. Salinity refers to salt level. Since the question is asking for the container with the greatest salinity, you are looking for the container with the least water (because it will be the saltiest out of all of them). Container 3 has the least water.
Hope this helps :)
3 has less water so its saltier
Which is the correctly balanced equation for the reaction of rust, Fe₂O3, and hydrochloric acid, HCI? O Fe₂O3 + HCI- FeCl₂ + H₂O O Fe₂O3 +3 HCI - 2 FeCl₂ + H₂O O Fe₂O3 +6 HCI - 2 FeCl3 + 3 H₂O O Fe₂O3 +3 HCI - 2 FeCl₂ + 3 H₂O
Answers
Answer:
Fe₂O3 +6 HCI - 2 FeCl3 + 3 H₂O
Explanation:
hope this helps.
The balanced equation obeys the law of conservation of mass. The balanced equation for the reaction of rust is Fe₂O₃ +6 HCI - 2 FeCl₃ + 3 H₂O. The correct option is C.
What is a balanced equation?A chemical equation in which the number of atoms of reactants and products on both sides of the equation are equal can be defined as the balanced chemical equation. The amount of products and reactants on both sides of the equation are equal.
The numbers which are added to balance a chemical equation are called the coefficients. They are added in front of the formulas.
The reaction of HCl with iron results in the formation of ferrous chloride (FeCl₂ or FeCl₃). When acid reacts with metal, it dissolves both the rust and the metal.
The balanced equation is:
Fe₂O₃ +6 HCI - 2 FeCl₃ + 3 H₂O
Thus the correct option is C.
To know more about balanced equation, visit;
https://brainly.com/question/15052184
#SPJ5
Determine the grams of sodium chloride produced when 10 g of sodium react with 10 grams chlorine gas according to the equation 2Na + Cl2 = 2 NaCl
Answers
Answer:
16 g
Explanation:
Step 1: Write the balanced equation
2 Na + Cl₂ ⇒ 2 NaCl
Step 2: Identify the limiting reactant
The theoretical mass ratio (TMR) of Na to Cl₂ is 46:71 = 0.65:1.
The experimental mass ratio (EMR) of Na to Cl₂ is 10:10 = 1:1.
Since EMR > TMR, Cl₂ is the limiting reactant
Step 3: Calculate the mass of NaCl produced
The theoretical mass ratio of Cl₂ to NaCl is 71:117.
10 g Cl₂ × 117 g NaCl/71 g Cl₂ = 16 g NaCl
For the following hypothetical reaction:
3A + 12B --> 3C
How many moles of B will you need to convert 5 moles of A into as many moles of C as possible?
Answers
Answer:
20
Explanation:
You need 4 times the amount of B, compared to a (there is a 3A:12B which reduces to 1:4), so 4 times 5 is 20.
Hope this helps!
Question 5(Multiple Choice Worth 4 points)
(06.03 MC)
The graph shows the changes in the phase of ice when it is heated.
Phase Change of Ice
C-
Temperature
(C)
B
A-
solid
liquid
25
gas
50 75 100
Time (min)
Which of the following temperatures describes the value of B?
O0 °C, because it is the melting point of ice.
O 100 °C, because it is the boiling point of water.
O Greater than 0 °C, because A represents the temperature at which ice melts.
O Greater than 100 °C, because A represents the temperature at which water evaporates
Answers
The temperatures that describes the value of B is A, 0 °C, because it is the melting point of ice.
What does the graph represent?The graph shows that the temperature of the ice is increasing as it is heated. The point at which the line changes from solid to liquid is the melting point of ice. This is the temperature at which the ice changes its phase from solid to liquid. The melting point of ice is 0 °C.
The other options are incorrect. The boiling point of water is 100 °C, and the temperature at which water evaporates is also 100 °C. The temperature at point A is greater than 0 °C, but it is not greater than 100 °C.
Find out more on temperatures here: https://brainly.com/question/4735135
#SPJ1
HELP! 10 points rewarded, plus, best answer gets BAINLIEST!!

Answers
Answer:
4.08 grams
Explanation:
Essentially, we're looking for the mass of HCl that "matches" 3.26 grams of magnesium hydroxide.
First, convert 3.26 grams of \(Mg(OH)_2\) into moles by dividing by the molar mass. The molar mass of \(Mg(OH)_2\) is 24.3 + 16 * 2 + 1 * 2 = 58.3 g/mol. So, 3.26 grams is equal to:
3.26 g ÷ 58.3 g/mol = 0.0559 mol \(Mg(OH)_2\)
Notice that from the chemical equation, magnesium hydroxide and hydrochloric acid (HCl) have a ratio of 1 to 2. In other words, for every 0.0559 moles of \(Mg(OH)_2\), there are 0.0559 * 2 = 0.112 moles of HCl.
Finally, convert moles of HCl to grams by multiplying 0.112 by the molar mass, which is 1 + 35.45 = 36.45 g/mol:
0.112 mol HCl * 36.45 g/mol = 4.08 g HCl
The answer is thus 4.08 grams.
~ an aesthetics lover
if you needed to produce 2.5 moles of CO2 for your volcano, how much baking soda (NaHCO3) should be used (anwser in grams)
Answers
Answer: Help
Explanation:
.
What will be the aproximate final volume of a solution prepared by diluting 25 mL of 8.25 M sodium hydroxide to a concentration of 2.40 M? A. 96 mL B. 25 mL C. 86 mL D.1.38 x 10^2 mL
Answers
The correct option is (C). The approximate final volume of a solution prepared by diluting 25 mL of 8.25 M sodium hydroxide to a concentration of 2.40 M is 86 mL (option C).
To find the final volume of the solution, we can use the formula:
C1V1 = C2V2
Where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Plugging in the values we know, we get:
(8.25 M) (25 mL) = (2.40 M) (V2)
Solving for V2, we get:
V2 = (8.25 M x 25 mL) / 2.40 M
V2 = 86.25 mL
Therefore, the approximate final volume of the solution prepared by diluting 25 mL of 8.25 M sodium hydroxide to a concentration of 2.40 M is 86 mL (option C).
Learn more about final volume at: https://brainly.com/question/22012954
#SPJ11
ultraviolet, visible, and infrared light are all examples of _____ radiation, which has the properties of both particles and ______.
Answers
ultraviolet, visible, and infrared light are all examples of electromagnetic radiation, which has the properties of both particles and waves.
What is infrared light?
Infrared light is a type of electromagnetic radiation that has a longer wavelength than visible light. It is invisible to the human eye, but can be detected by special cameras and sensors. It is used in many applications, including night vision, thermal imaging, and remote control.
Therefore, ultraviolet, visible, and infrared light are all examples of electromagnetic radiation, which has the properties of both particles and waves.
To learn more about infrared light
Here: https://brainly.com/question/30551457
#SPJ4
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
Explain why uranium must be enriched to be used in a nuclear power plant.
Answers
Answer:
The nuclear fuel used in a nuclear reactor needs to have a higher concentration of the U 235 isotope than that which exists in natural uranium ore. U235 when concentrated (or "enriched") is fissionable in light-water reactors (the most common reactor design in the USA).
Explanation:
Boron has an average atomic mass of 10.81. One isotope of boron has a mass of 10.012938 and a relative abundance of 19.80 percent. The other isotope has a relative abundance of 80.20 percent.
What is the mass of that isotope? Report to two decimal places.
Answers
The mass of the other boron isotope is 11.0 u.
What is the mass of the other isotope?We know that isotopes do not have the same mass number but they do have the same atomic number because they come from the same element as we know.
Now we can see that;
Average atomic mass = Sum of the weighted masses of the isotopes.
Let the unknown mass be x
10.81 = (10.012938 * 0.198) + (x * 0.802)
10.81 = 1.98 + 0.80x
x = 10.81 - 1.98/0.80
x = 11.0
Thus the other isotope is found to have a mass of about 11.0 .
Learn more about atomic mass:https://brainly.com/question/17067547
#SPJ1
what is the reaction between phosphate ion (PO4 3-) and hydronium ion (H3O+)
Answers
Answer:
H3O+(aq) + PO43−(aq) ⇌ HPO42−(aq) + H2O(l)
Notice that the total charge (2-) is the same on both sides of the equation, and that this is reaction can take place in both directions.
Explanation:
i hope it helps uh..!!mark me as brainliest ♥️Which one of the following reagents adds a methyl group by conjugate (1,4-addition) to an a,B-unsaturated ketone or aldehyde?
A. LiCu(CH3)2
B. CH3MgBr
C. Hg(O2CCH3)2
D. CH3Li
Answers
Option B. \(CH_3MgBr\) is the reagent adds a methyl group by conjugate (1,4-addition) to an a,B-unsaturated ketone or aldehyde
\(CH_3MgBr\), known as methylmagnesium bromide or Grignard reagent, adds a methyl group by conjugate (1,4-addition) to an α,β-unsaturated ketone or aldehyde. Grignard reagents are organometallic compounds that are highly reactive nucleophiles and are commonly used in organic synthesis.
The addition of the methyl group to the α,β-unsaturated ketone or aldehyde occurs in a conjugate manner, meaning that the addition takes place at the β-carbon (the carbon adjacent to the carbonyl carbon) of the unsaturated system. This leads to the formation of a new carbon-carbon bond and results in the addition of the methyl group.
To know more about Grignard Reagent:
https://brainly.in/question/9348033
#SPJ11
Decane (C10H22) is used in diesel. The combustion for decane follows the equation: 2 C10H22 + 31 O2 à 20 CO2 + 22 H2O. Calculate the amount, in grams, of water (H2O) produced by the combustion of 568 grams of decane (C10H22) with 2976 grams of oxygen gas (O2). 692 792 892 992
Answers
The mass of water produced is 792 grams by the combustion of 568 grams of decane.
Given:
Combustion of 568 grams of decane with 2979 grams of oxygen.
\(2 C_{10}H_{22 }+ 31 O_2 \rightarrow 20 CO_2 + 22 H_2O\)
To find:
The mass of water produced by combustion of 568 grams of decane.
Solution:
Mass of decane = 568 g
Moles of decane :
= \(\frac{568 g}{142 g/mol}=4 mol\)
Mass of oxygen gas = 2976 g
Moles of oxygen gas:
= \(\frac{2976 g}{32 g/mol}=93 mol\)
\(2 C_{10}H_{22 }+ 31 O_2 \rightarrow 20 CO_2 + 22 H_2O\)
According to reaction, 2 moles of decane reacts with 31 moles of oxygen, then 4 moles of decane will react with:
\(=\frac{31}{2}\times 4mol=62\text{ mol of}O_2\)
But according to the question, we have 93.0 moles of oxygen gas which is more than 62 moles of oxygen gas.
So, this means that oxygen gas is present in an excessive amount. Which simply means:
Oxygen gas is an excessive reagent.Decane is a limiting reagent.Decane being limiting reagent will be responsible for the amount of water produced after the reaction.According to reaction, 22 moles of water is produced from 2 moles of decane, then 4 moles of decane will produce:
\(=\frac{22}{2}\times 4mol=44\text{mol of }H_2O\)
Mass of 44 moles of water ;
\(=44mol\times 18g/mol=792g\)
792 grams of water is produced by the combustion of 568 grams of decane.
Learn more about limiting reagent and excessive reagent here:
brainly.com/question/14225536?referrer=searchResults
brainly.com/question/7144022?referrer=searchResults
The kinetic energy of a moving object depends on its mass and its
a. volume.
b. velocity.
c. distance.
d. acceleration.
Answers
Which model could represent a neutral atom of nitrogen

Answers
Answer:
The answer is c.1. The atomic number of nitrogen is 7.
The electron arrangement of nitrogen is 2.5. With that said, atom 1 resembles the neutral atom of nitrogen because the innermost shell of the atom contains 2 electrons while the outermost shell of the atom contains 5 electrons.
The model that could represent a neutral atom of nitrogen is the first one. Thus, the correct option is C.
What is a Nitrogen atom?A nitrogen atom may be defined as a chemical element with an atomic number of 7 and an atomic mass of 14. It contains seven protons in its nucleus.
Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table. Being neutral, the number of protons that it has in its nucleus must be equal to that of the surrounding electron. Hence, the number of electrons are seven.
These seven electrons are only visible in the first model. Thus, the correct option for this question is C.
To learn more about Nitrogen, refer to the link:
https://brainly.com/question/15373263
#SPJ5
A particle ‘A’ of mass of 2.0 kg has charge 1.2 μC deposited on it. Determine the ratio of electric and gravitational force between ‘A’ and ‘B’ if mass of ‘B’ is 1.5 kg and charge on it is 0.92 μC. distance between particle ‘A’ and ‘B’ is 4.8 m.
Answers
Answer: The correct answer is 4.956 * 10^7.
Explanation:
For Electrostatic force,
Given qA =1.2 × 10∧-6 C (Since 1 micron = 10∧-6)
qB=0.92 ×10∧-6 C Since 1 micron = 10∧-6)
r = 4.8m
Electrostatic force = (K×qA×qB)÷r∧2 where K is Coulomb's constant or electrostatic constant =8.98755×10∧9
Therefore Electrostatic energy =(8.98755×10∧9×1.2×0.92×10∧-12)÷4.8∧2
=0.00043065 N ················ eq1
Now for Gravitational force,
mA=2Kg ,mB=1.5Kg ,r=4.8m,G is Gravitational constant =6.67408 × 10-11 N m2 kg-2
Gravitational force=(G×mA×mB)÷r∧-2
=(6.67408 × 10-11 ×2×1.5)÷4.8∧-2
=0.869021875 ×10∧-11 N...............eq2
Ratio of electric and gravitational force between ‘A’ and ‘B’ = eq1÷eq2
=49555714.5785
Electrostatic force occurs due to interaction either between like charges that is either between positive-positive or negative negative charges or between unlike charges like positive-negative. Its strength depends on the charges and the distance between the charges which decreases as the distance increases.
Gravitational force occurs due to the fact every particle attracts each and every other particle in the universe. Its strength depends on the mass and the distance between the particles which decreases as the distance increases.
For further reference on gravitational and Electrostatic refer:
https://brainly.com/question/24783651
compare the size of ions to the size of atoms from which they form
Answers
Cations are always smaller than the atoms from which they form. Anions are always larger than the atoms from which they form. Ions are usually bigger than the atoms from which they are formed.
When an atom receives or loses electrons, the atom's electron configuration changes, resulting in a net positive or negative charge.
This net charge expands the electron cloud surrounding the nucleus, making the ion bigger in size than the neutral atoms from which it arose. When a metal atom loses one or more electrons to create a cation, it shrinks in size because the positive charge of the nucleus pulls the remaining electrons more strongly.
When a nonmetal atom obtains one or more electrons to create an anion, it normally expands in size.Because of the increasing amount of electrons, the electron cloud surrounding the nucleus grows. It should be noted that this comparison is not absolute and is dependent on the individual factors involved. Some ions are smaller than their neutral atom counterparts, while others are similar in size.
learn more about atoms here:
https://brainly.com/question/29695801
#SPJ4
The complete question is:
Compare the size of ions to the size of atoms from which they form.
8. How many moles are in 4.06x1025 atoms of Iron?
Answers
bjkbkjbvnbmnbkbggvjhkbvhyvhbvkhvkhvkhvyhvhvhkvkhvik
To convert moles from atoms, moles is divied by Avagodro's number to get moles. The mole is 67.40 moles.
What is Avagodro’s number?
Avagodro's number is the every moles contains of 6.023x10^23 atoms.
The mole is used to measure the quantity of amount of substance. It’s the number of elementary entities of a given substance that are present in a given sample
To find out the number of atoms using the following formula.
moles x Avagodro's number = number of atoms
moles = Number of atoms/Avagodro's number
thus, moles = 67.40 moles
To find more about Mole, refer the link below;
https://brainly.com/question/26416088.
#SPJ2
What is the mass number and atomic number of Ca0
Answers
Answer:
56.0774g/mol
Explanation:
hope you like it ☺️
phospholipases are important to i. sphingomyelin head group exchange ii. eicosanoid biosynthesis iii. generation of some second messengers iv. fatty acid liberation
Answers
Phospholipases play a crucial role in various cellular processes, including sphingomyelin head group exchange, eicosanoid biosynthesis, generation of some second messengers, and fatty acid liberation.
i. Sphingomyelin head group exchange: Phospholipases participate in the hydrolysis of sphingomyelin, a type of phospholipid, releasing the phosphorylcholine head group. This process is essential for the turnover and remodeling of cellular membranes, as well as for regulating cell signaling pathways.
ii. Eicosanoid biosynthesis: Phospholipases, particularly phospholipase A2, are involved in the release of arachidonic acid from membrane phospholipids. Arachidonic acid serves as a precursor for the synthesis of eicosanoids, including prostaglandins, leukotrienes, and thromboxanes, which are important mediators of inflammation, immune responses, and other physiological processes.
iii. Generation of some second messengers: Phospholipases, such as phospholipase C, contribute to the generation of second messengers like diacylglycerol (DAG) and inositol trisphosphate (IP3). Upon activation, phospholipase C hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into DAG and IP3, which regulate intracellular calcium levels and protein kinase C activation, influencing cellular processes like signal transduction and gene expression.
iv. Fatty acid liberation: Phospholipases mediate the hydrolysis of fatty acids from phospholipids, releasing free fatty acids. This process is crucial for providing a source of fatty acids that can be utilized for energy production, membrane synthesis, or as signaling molecules.
In summary, phospholipases play diverse roles in sphingomyelin head group exchange, eicosanoid biosynthesis, generation of second messengers, and fatty acid liberation. Their activities contribute to cellular signaling, membrane dynamics, and the regulation of various physiological processes.
Know more about Phospholipases here:
https://brainly.com/question/14849157
#SPJ11