what can you infer about an ion that has a negative charge?
I NEED HELP FAST FOR MY TEST!!!
Answers
Answer:
It has more electrons than protons. It is an anion.
Related Questions
26 grams of 2,2 dimethyl hexane( C8H16) under goes combustion. How
much space would the CO2 gas occupy at 26 Celsius and 101.75 kPa?
Answers
Answer: The \(CO_{2}\) gas occupies a space of 5.57 L at 26 Celsius and 101.75 kPa.
Explanation:
Given: Mass = 26 g
Pressure = 101.75 kPa
Convert kPa into atm as follows.
\(1 kPa = 0.00986923 atm\\101.75 kPa = 101.75 kPa \times \frac{0.00986923 atm}{1 kPa}\\= 1 atm\)
Temperature = \(26^{o}C = (26 + 273) K = 299 K\)
Now, moles of a substance is defined as mass of substance divided by its molar mass.
As molar mass of 2,2 dimethyl hexane is 114.23 g/mol. So, its number of moles are calculated as follows.
\(No. of moles = \frac{mass}{molar mass}\\= \frac{26}{114.23 g/mol}\\= 0.227 mol\)
Formula used to calculate the volume is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
\(PV = nRT\\1 atm \times V = 0.227 mol \times 0.0821 L atm/mol K \times 299 K\\V = 5.57 L\)
Thus, we can conclude that the \(CO_{2}\) gas occupies a space of 5.57 L at 26 Celsius and 101.75 kPa.
what is an example of a solid solute that does not follow the general trend of increasing solubility with increasing temperature?
Answers
In the following question, One example of a solid solute that does not follow the general trend of increasing solubility with increasing temperature is Cerium (III) sulfate tetrahydrate (Ce2(SO4)3.4H2O).
Why does cerium sulfate tetrahydrate not follow the general trend of increasing solubility with increasing temperature? This is due to the fact that the solubility of Ce2(SO4)3.4H2O decreases as the temperature rises. Cerium (III) sulfate tetrahydrate is a white or colourless crystalline solid that is moderately soluble in water, with a solubility of 8.3 grams per litre at 25 degrees Celsius. It is a rare compound that is difficult to come by. Ce2(SO4)3.4H2O is a compound that exists in several crystalline forms. It has a high melting temperature and decomposes upon heating. At low temperatures, Ce2(SO4)3.4H2O is sparingly soluble. When the temperature rises, the solubility drops, reaching its lowest value at 90°C, after which it starts to rise slowly. The main reason why the solubility of Ce2(SO4)3.4H2O decreases as the temperature increases are due to the endothermic reaction that occurs when the compound dissolves in water. Heat is absorbed when a solute is dissolved in a solvent in an endothermic reaction. The solubility of endothermic solids, such as cerium (III) sulfate tetrahydrate, decreases as the temperature rises.
For more such questions on Cerium
https://brainly.com/question/9448870
#SPJ11
How many grams are there in 2.8 * 10 ^ 24 molecules of Li 2 O?
Answers
First, we must know Avogadro's number:
1 mol of any substance = 6.02x10^23 formula units (ions, atoms, molecules, etc)
Also, we can say this:
1 mol of Li2O = its mass expressed in grams
(from Li2O molar mass = 30 g/mol approx.)
Now,
1 mol Li2O = 30 g = 6.02x10^23 molecules Li2O
30 g Li2O ----------- 6.02x10^23 molecules Li2O
x ----------- 2.8x10^24 molecules Li2O
x = 140 g approximately
Answer: 140 g
Group I (the alkali metals) includes lithium (Li), sodium (Na), and potassium (K). These elements have similar chemical properties because they have the same _______________.
Answers
amount of electrons on their outer shell: 1 electron.
These elements have similar chemical properties because they have the same valence electrons.
What is valence electrons?A valence electron is just a negatively charged particle that could be transferred to and is found in the atom's outermost shell.
What is elements?A pure substance made up entirely of atoms with the same number of protons in respective nuclei is known as an element.
Therefore, these elements have similar chemical properties because they have the same valence electrons.
To know more about valence electrons.
https://brainly.com/question/12717954.
#SPJ2
How many atoms are there in the repeating peptide backbone units of proteins? Select one: a. 1 b. 2 c. 3 d. 4 e. 5
Answers
The repeating peptide backbone units of proteins contain 3 atoms. By understanding the structure of the "backbone" for peptides and proteins.
The structure of a peptide may be written very simply without illustrating the entire amide synthesis step.
The N-H 2, CH, C double bond O; N-H 2, CH, C double bond O; etc. repeating units make up the peptide backbone.
Nothing more than the use of the amide synthesis process results in the creation of peptides. The amide bond in peptides is often formed in the same sequence that the amino acids are listed. An amino acid's amine end (N terminal) is always on the left and its acid end (C terminal) is always on the right. The diagram on the left illustrates how to write the reaction between glycine and alanine to create the dipeptide glyclalanine. A water molecule is created when hydrogens (red) on the amine and oxygen (red) from the acid combine. The amide bond is created by the joining of the carboxyl oxygen (green) with the amine nitrogen (green).
Learn more about Peptide here:
https://brainly.com/question/30332053
#SPJ4
A package of chocolate instant pudding contains 2640 mg of sodium. how many grams of sodium are in the pudding?
Answers
Answer:
2.640
Explanation:
Rounding to the nearest whole number would be 3 if that is needed.
Which ion is a cation? A. Ca2+ B. Cl2 C. S2- D. Br-
Answers
Answer:
Ca2+ is cation
Explanation:
Because cation contain positive charge.
Which describes the law of conservation of matter?
O A. Chemical reactions cannot happen.
B. Atoms cannot be created or destroyed in a reaction.
OC. Atoms are not involved in chemical reactions.
OD. Molecules cannot change into other molecules during a reaction.
SUBMIT
Answers
Answer:
answer is B
mark me brainliest
follow
What is the conjugate acid of HSO4- in the reaction below? H2 SO4 (aq) + H2 O(l) rightleftharpoons H3 O+(aq) + HSO 4 -(aq)
A. SO 4 ^ 2-
B. H 2 O
C. H 3 O^ +
D. H 2 SO 4
Answers
Answer:
When H2O acts as a base, it gains one H, which forms its conjugate acid, H30. ... Conjugate acid-base pairs: H2SO4/HSO4 andH3O+/H2O ... CO32-(aq) + H2O(1) 2 HCO3 (aq) + OH(aq).
The conjugate acid of HSO4- in the reaction is H2SO4.
The conjugate acid of a particular base is obtained by adding a proton to it.
Now we have the reaction; H2SO4 (aq) + H2O(l) -----> H3O+(aq) + HSO4^-(aq). We have to look at the reaction carefully and find out which specie can be obtained from HSO4- by adding a proton.
Clearly, the specie that we are looking for is the same specie from which a proton was removed to yield HSO4-.
Through examination of the reaction shows that this specie must be H2SO4.
Learn more: https://brainly.com/question/15220688
1. Based on the data set and the true measured value being 28km.
A. Is this data accurate, precise, or both?
B. What is the average?
29km
28.3km
28.2km
27.22km
28.1257km
28km
27.8km
28.6km
28km
27.777777777km
Thank you!
Answers
Who is the kingdom of plants?
Answers
Answer:
i think you mean what is the kingdom of plants, but its "Kingdom Plantae".
Kingdom Plantae traits: eukaryotic/have nucleus, multicellular and autotrophic. (they photosynthesis)
2nd largest kingdom
Answer:
Introduction: All the plants are placed in the Kingdom- Plantae, according to the five-kingdom classification by R.H. Whittaker. The Kingdom- Plantae consists of multicellular plants with eukaryotic organization and chlorophyllous cells.
Explanation:
What is the kingdom for plants called?
What are the 3 plant kingdoms?
Kingdom Plantae Organisms
Ferns: They fall under the division Pteridophyta and are known to have vascular tissue. ...
Mosses: They fall under the division Bryophyta and have no vascular system. ...
Cone-bearing plants: They fall under the division Spermatophyta, sub-division gymnosperms
A compound is 47.4% by mass carbon, 8.00% by mass hydrogen, 10.6% by mass oxygen, 21.2% by mass sulfur, and 12.6% by mass fluorine. What is the empirical formula?
Answers
A compound's chemical formula describes the proportions (ratios) of its constituent parts rather than the number or arrangement of atoms in the complex.
Is hydrogen water safe to consume?Drinking hydrogen water is not thought to be dangerous by experts. They are unsure, though, whether its advantages outweigh those of drinking regular water or being hydrated in general. A life-threatening hyponatremia can result from excessive water consumption.
Why is hydrogen ineffective as a fuel?However, it is not used as residential fuel for a number of reasons: The cost of manufacturing hydrogen is high, and it is not always accessible. In comparison to other gases, hydrogen is harder to come by in the environment.
To know more about Hydrogen visit:
https://brainly.com/question/28937951
#SPJ1
Calculate the [h ] for a 0. 0473 m solution of barium hydroxide, ba(oh)2 assuming complete dissociation of the compound
Answers
The hydrogen ion concentration ([h]) for a 0.0473 m solution of Barium hydroxide, \(Ba(OH)2\) assuming complete dissociation of the compound is 0.0946 mol/L.
The symbol [h] typically refers to the hydrogen ion concentration in a solution.
The balanced equation for the solution of barium hydroxide is
\(Ba(OH)_{2} (s)\) → \(Ba_{2}\)(aq) + 2OH-(aq)
Here \(Ba(OH)_{2}\) dissolves completely. The concentration of barium hydroxide and OH- will be equal to the concentration \(Ba(OH)2\) originally added to the solution
The OH- concentration will be calculated as:
[OH-] = 2 × 0.0473 mol/L
[OH-]= 0.0946 mol/L
[h] = [H+] = [OH-] = 0.0946 mol/L
Therefore, we can conclude that the hydrogen ion concentration ([h]) is 0.0946 mol/L.
To learn more about Barium hydroxide
https://brainly.com/question/31327057
#SPJ4
Chemical treatment (of hazardous waste) fefers to the treatment methods that are used io elfect the complete brealutovin of hazardour wase in
Answers
Chemical treatment of hazardous waste refers to the treatment methods that are used to effect the complete breakdown of hazardous waste. The hazardous waste is chemically processed in chemical treatment to make it less harmful and less toxic.
There are several different chemical treatment methods used to treat hazardous waste, including oxidation, reduction, neutralization, and precipitation. In oxidation, the waste is treated with oxidizing agents to convert the waste into a less harmful form. In reduction, the waste is treated with reducing agents to convert the waste into a less harmful form. In neutralization, an acid or base is added to the waste to neutralize its pH.
In precipitation, a chemical is added to the waste to cause it to precipitate out of solution. Chemical treatment is one of several methods used to manage and dispose of hazardous waste in an environmentally responsible manner.
To know more about Chemical treatment visit:
https://brainly.com/question/14690736
#SPJ11
How many grams of iron (III) oxide can be produced from 2.50 g of oxygen reacting with iron, according to the
following equation?
4 Fe (s) + 3 02 (g) -->2 Fe₂O3(s)
Answers
3 moles of oxygen gives 2 moles of the product. Then, 2.50 g or 0.07 moles of oxygen gas will give, 0.04 moles or 14.8 g of Fe₂O₃.
What is Fe₂O₃ ?Metals are easily reactive towards atmospheric oxygen and they form their oxides. Fe reacts with oxygen to form one of its oxide in the + 3 oxidation state that is Fe₂O₃.
Here, 3 moles of oxygen gives 2 moles of the oxide.
molar mass of oxygen gas = 32 g/mol
no.of moles in 2.5 g = 2.5 /32 = 0.07 moles.
0.07 moles produce, 0.07 × 2 /3 = 0.04 moles.
molar mass of Fe₂O₃ = 319.2 g.
then, mass of 0.04 moles = 0.04 × 319.2 = 14.8 g.
Therefore, 2.5 g of oxygen gas will give 14.8 g of the product.
Find more on Fe₂O₃:
https://brainly.com/question/24236942
#SPJ1
Choose the element with the highest first ionization energy from each of the following pairs. Kr or Br Br Kr Mg or Ca Ca Mg P or As P As P or Su Su P
Answers
The elements with the higher first ionization energy in each pair are Kr, Ca, As, and S.
The first ionization energy is the amount of energy required to remove the outermost electron from an atom in its gaseous state. Elements with higher effective nuclear charge, i.e., a greater number of protons in the nucleus, will require more energy to remove the outermost electron, and hence have higher first ionization energies.For each of the pairs given, the element with the higher first ionization energy is:Kr or Br: Kr has a higher first ionization energy than Br. This is because Kr has a greater effective nuclear charge than Br, due to the greater number of protons in its nucleus, which increases the attractive force between the electrons and the nucleus.Mg or Ca: Ca has a higher first ionization energy than Mg. This is because Ca has a greater effective nuclear charge than Mg, due to the greater number of protons in its nucleus, which increases the attractive force between the electrons and the nucleus.P or As: As has a higher first ionization energy than P. This is because As has a greater effective nuclear charge than P, due to the greater number of protons in its nucleus, which increases the attractive force between the electrons and the nucleus.P or S: S has a higher first ionization energy than P. This is because S has a greater effective nuclear charge than P, due to the greater number of protons in its nucleus, which increases the attractive force between the electrons and the nucleus.In summary, the elements with the higher first ionization energy in each pair are Kr, Ca, As, and S.For more such question on ionization energy
https://brainly.com/question/30831422
#SPJ11
DUE IN 11 MINS Convert the following to mass in grams1.75 x 1023 atoms Pb
Answers
The mass of 1.75 x 10²³ atoms of Pb is approximately 60.1 grams. This will took place in a amount of 11 minutes only.
To convert the given number of atoms of Pb to grams, we need to use the molar mass of Pb.
The atomic mass of Pb is 207.2 g/mol (rounded to one decimal place). This means that one mole of Pb atoms weighs 207.2 grams.
To find the mass of 1.75 x 10²³ atoms of Pb, we can use the following steps:
Calculate the number of moles of Pb atoms in 1.75 x 10²³ atoms:
(1.75 x 10²³ atoms) / (6.022 x 10²³ atoms/mol) = 0.290 moles
Convert moles to grams using the molar mass of Pb:
0.290 moles x 207.2 g/mol = 60.1 grams
Therefore, 1.75 x 10²³ atoms of Pb is equivalent to 60.1 grams.
To know more about mass please refer: https://brainly.com/question/15859030
#SPJ1
Question - What is the mass, in grams, of 1.75 x 10^23 atoms of lead (Pb), in due of 11 minutes?
What type of reaction is depicted in the equation below?
X + Y - XY
O Combustion
O Decomposition
Synthesis
O Double Replacement
Answers
Answer:XY X +Y
Explanation:
general reaction represents a decomposition reaction where a molecule is “broken” in two separate molecules.
if 67.9 ml of lead(ii) nitrate solution reacts completely with excess sodium iodide solution to yield 0.699 g of precipitate, what is the molarity of lead(ii) ion in the original solution?
Answers
The molarity of lead(II) ion in the original solution is 0.0112 M.
The balanced chemical equation for the reaction between lead(II) nitrate and sodium iodide is:
Pb(NO3)2 + 2 NaI → PbI2 + 2 NaNO3
From this equation, we can see that 1 mole of lead(II) nitrate reacts with 2 moles of sodium iodide to produce 1 mole of lead(II) iodide precipitate.
First, let's calculate the number of moles of lead(II) iodide precipitate formed in the reaction:
mass of PbI2 = 0.699 g
The molar mass of PbI2 is 461.01 g/mol, so the number of moles of PbI2 formed is:
moles of PbI2 = 0.699 g ÷ 461.01 g/mol = 0.001515 mol
Since the reaction is between lead(II) nitrate and sodium iodide, the number of moles of lead(II) nitrate present in the solution can be calculated using the stoichiometry of the balanced chemical equation:
moles of Pb(NO3)2 = 1/2 × moles of PbI2 = 0.0007575 mol
Now, we can calculate the molarity of lead(II) ion in the original solution using the volume of the lead(II) nitrate solution:
volume of Pb(NO3)2 solution = 67.9 mL = 0.0679 L
Molarity of Pb2+ = moles of Pb(NO3)2 / volume of Pb(NO3)2 solution
Molarity of Pb2+ = 0.0007575 mol / 0.0679 L = 0.0112 M
Therefore, the molarity of lead(II) ion in the original solution is 0.0112 M.
To know more about Molarity:
https://brainly.com/question/9558971
#SPJ4
Answer question will give brainliest

Answers
Explanation:
Elastic Potensial energy is what causes a ball to bounce or rebound because it is transformed into kinetic energy, which is then used to bring the ball back up.
What is the molarity of a solution containing 5. 0 moles of solute in 469 mL of a soltution
Answers
molarity of a solution containing 5. 0 moles of solute in 469 mL of solution is 10.66 mol/L
We know that the Molarity of a given solution is -
Molarity = n/V-------------------(i)
where
n= number of moles
V = Volume of solution in liters
now as per the question-:
number of moles n = 5 moles
volume of solution V= 469 ml or 0.469 ml { convert volume given in ml to L }
putting the values in equation (i)
\(Molarity =\frac{5}{0.469}\)Molarity = 10.66 mol/L
Therefore Molarity of the given solution is 10.66 mol/L
For more information on molarity
https://brainly.com/question/30404105
What’s the answer? I need an answer as soon as possible please!

Answers
Answer:
1, 1, 2
Explanation:
1, 1, 2
The coefficients for the N2O5 and H2O are technically 1; if you must provide a value for those reactants, then that would be it.
2co(g)+o2(g)⇌2co2(g),if a sample of 70 g of carbon monoxide was reacted with 32 g of oxygen how many moles of carbon dioxide would be produced
Answers
When 70 g of carbon monoxide was reacted with 32 g of oxygen, 0.5001 moles of carbon dioxide would be produced.
We can start by using the given amounts of reactants to determine which is the limiting reactant and calculate the theoretical yield of carbon dioxide that can be produced.
The balanced chemical equation tells us that two moles of CO react with one mole of O2 to produce two moles of CO2:
2 CO(g) + O2(g) → 2 CO2(g)
The molar mass of CO is 28.01 g/mol, and the molar mass of O2 is 32.00 g/mol. Using these values, we can calculate the number of moles of each reactant:
Number of moles of CO = mass ÷ molar mass = 70 g ÷ 28.01 g/mol ≈ 2.498 mol
Number of moles of O2 = mass ÷ molar mass = 32 g ÷ 32.00 g/mol = 1.000 mol
From the balanced equation, we see that 1 mole of O2 reacts with 2 moles of CO to produce 2 moles of CO2.
Therefore, the 1.000 mole of O2 can react with 0.5000 moles of CO (2.498 mol ÷ 2), which is the limiting reactant.
The theoretical yield of CO2 that can be produced is:
0.5000 mol CO × (2 mol CO2 ÷ 2 mol CO) × (44.01 g/mol CO2) = 22.01 g CO2
Therefore, 22.01 g of carbon dioxide would be produced.
Number of moles of CO2 produced = mass ÷ molar mass = 22.01 g ÷ 44.01 g/mol ≈ 0.5001 mol
Therefore, approximately 0.5001 moles of CO2 would be produced.
To know more about moles, refer here:
https://brainly.com/question/11858379#
#SPJ11
Look at your gumdrop model of a water molecule. If you had several of those water molecules, and you pulled the gumdrops apart, what other molecules could you make? Explain your answer.
Answers
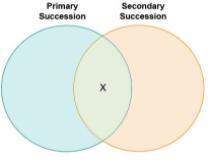
his Venn diagram is being used to compare primary and secondary successions.
Which statement belongs in the region marked X?
occurs only in cases of volcanic activity
changes occur gradually
begins when lichens populate an area
results in a reduction of the number of species
Answers
The statement that belongs in the region marked X in terms of primary and secondary succession is B. changes occur gradually.
How to described primary and secondary successions ?Primary succession occurs in areas where there is no existing soil or organic matter, such as on newly formed volcanic islands, sand dunes, or glacial moraines.
Secondary succession occurs in areas where there has been a disturbance or disruption of an existing ecosystem, such as from a fire, flood, or human activity. The region marked x would therefore include no change when both successions intersect.
Find out more on primary succession at https://brainly.com/question/520254
#SPJ1
What is the difference between fission and fusion? What are some problems to overcome with each?
Answers
Answer:
Explanation:
The main difference between these two processes is that fission is the splitting of an atom into two or more smaller ones while fusion is the fusing of two or more smaller atoms into a larger one.
Fission means splitting 1 atom into more atom
Fusion means Combination of 2 atom or more atom.
An atom:
A. Is the smallest whole unit of matter
B. Can form one or more elements
C. Is the smallest whole unit of matter with the properties of a specific element
D. All of the above
Answers
Answer:
The answer is A: Is the smallest whole unit of matter
How many isotopes does Potassium have?
Here is my work:

Answers
Potassium have three isotopes.
How many different isotopes are there overall?254 stable isotopes are known. Certain substances can only exist in unstable states (for example, uranium).
The stable potassium isotope has a nuclear spin of 3/2, a relative atomic mass of 38.963707, and a natural abundance of 93.3 atoms.
We must compute for the various atomic weights of the isotopes since potassium has three distinct isotopes: Potassium-39 has an atomic mass of 39 and 19 protons. Therefore, the number of neutrons is 39 – 19, or 20.
If potassium has an atomic mass of 39.0983, the average is obviously highly biassed in favour of potassium-39, making potassium-39 the isotope with the greatest abundance.
learn more about isotope refer
https://brainly.com/question/14220416
#SPJ13
When matter undergoes a physical change, mass is
a
always conserved.
b
sometimes conserved.
c
never conserved.
Answers
A baseball is thrown a distance of 20 meters. What is its speed if it takes 1 second to cover the distance?