Answers
Answer:
Identifies the number of protons a single atom of the element contains.
Explanation:
The atomic number helps people identify elements according to the number of protons one atom of the element has. It essentially defines the element.
While having a neutral charge, it also provides the number of electrons the element has (in one atom).
Related Questions
Can I get some help for these chemistry questions .

Answers
1. C6H10O5 + 6O2 → 6CO2 + 5H2O
2. MgO + H2O → Mg(OH)2
3. 151.2444 grams of carbon dioxide are required to produce 3.44 moles of carbonic acid in the blood.
1. The complete, balanced chemical reaction for the burning of cellulose (C6H10O5), or wood, is:
C6H10O5 + 6O2 → 6CO2 + 5H2O
This reaction represents the combustion of cellulose in the presence of oxygen, resulting in the formation of carbon dioxide (CO2) and water (H2O).
2. The balanced equation for the reaction between magnesium oxide (MgO) and water (H2O) to produce magnesium hydroxide (Mg(OH)2) is:
MgO + H2O → Mg(OH)2
According to the equation, 1 mole of magnesium oxide reacts with 1 mole of water to produce 1 mole of magnesium hydroxide. Therefore, if 2.55 moles of magnesium oxide react, the same number of moles of magnesium hydroxide will be produced.
3. The balanced equation for the reaction between carbon dioxide (CO2) and water (H2O) to form carbonic acid (H2CO3) is:
CO2 + H2O → H2CO3
According to the equation, 1 mole of carbon dioxide reacts with 1 mole of water to produce 1 mole of carbonic acid. Therefore, if 3.44 moles of carbonic acid are produced, the same number of moles of carbon dioxide is required.
To calculate the mass of carbon dioxide, we need to know its molar mass, which is approximately 44.01 g/mol. Therefore, the mass of carbon dioxide required to produce 3.44 moles of carbonic acid is:
Mass = Moles × Molar mass
Mass = 3.44 moles × 44.01 g/mol
Mass = 151.2444 g
Therefore, 151.2444 grams of carbon dioxide are required to produce 3.44 moles of carbonic acid in the blood.
For more such qeustions on carbon dioxide visit:
https://brainly.com/question/21256960
#SPJ8
Cyclopentene decomposes at 528 K as shown in the following reaction: C5H8 (g) ⇒C5H6 (g) + H2 (g)
Time (s) [C5H8] (mol L-1 )
0 0.02
20 0.0189
50 0.0173
100 0.0149
200 0.0112
300 0.0084
400 0.0063
500 0.0047
700 0.0027
1000 0.0011
Find the reaction order
Answers
Answer:
rate=k×[C5H8]^n
k is the rate const.
when the rate is calculated using a few of the above data, (rate=change in concentration/time period) it can be observed that the rate is constant
therefore order of the reaction is 0
What is the maximum number of electrons possible with n = 1 in an atom?
Answers
The maximum number of electrons that can exist in the first energy level, n = 1, is 2.
What is the first energy level?The first energy level can hold a maximum of 2 electrons, and it is the closest energy level to the nucleus.
The first energy level consists of only one sub-shell, which is the s sub-shell, and it contains only one spherical orbital.
Therefore, according to the Pauli Exclusion Principle, which states that no two electrons can have the same set of four quantum numbers, the first energy level can hold a maximum of 2 electrons, with one electron occupying the 1s orbital, and the other occupying the 1s* orbital (also known as the anti-bonding orbital).
Learn more about electrons at:
https://brainly.com/question/20380276
#SPJ1
8.50g of CuO when heated in a current of dry hydrogen gas gave 6.58g of copper and 2.16g of wate Calculate the proportion of oxygen to hydrogen by mass in water.
Answers
The proportion of oxygen to hydrogen by mass in the water is 8 : 1
Data obtained from the question Mass of CuO = 8.50 gMass of Cu = 6.58 gMass of water = 2.16g Proportion =? How to determine the mass of O in water 1 mole of water (H₂O) = (2×1) + 16 = 18 gMass of O in water = 16 g
18 g of water contains 16 g of O.
Therefore,
2.16 g of water will contain = (2.16 × 16) / 18 = 1.92 g of O
How to determine the mass of H in water Mass of water = 2.16 gMass of O in water = 1.92 gMass of H =?Mass of H = mass of water – mass of O
Mass of H = 2.16 – 1.92
Mass of H = 0.24 g
How to determine the proportion of oxygen to hydrogenMass of O = 1.92 gMass of H = 0.24 gProportion =?Proportion = oxygen / hydrogen
Proportion = 1.92 / 0.24
Proportion = 8 : 1
Learn more about mass composition:
https://brainly.com/question/11617445
How did the mass change when the copper coin was made to look silver?
a .It stayed the same.
b. It decreased.
c . It increased.
Answers
We have that The the mass change when the copper coin was made to look silver is an increase in mass
Correct option C
It increased.
It is important to note that the copper coin after its cutting into shape will have a specific mass or weight and the silver coating solution will also have a net value of mass or weight
Therefore
The the mass change when the copper coin was made to look silver is an increase in mass
Correct option C
It increased.
For more information on this visit
https://brainly.com/question/17756498?referrer=searchResults
Classify each of the following word equations as a synthesis, decomposition, single displacement, or double displacement reaction.
Will give brainliest.
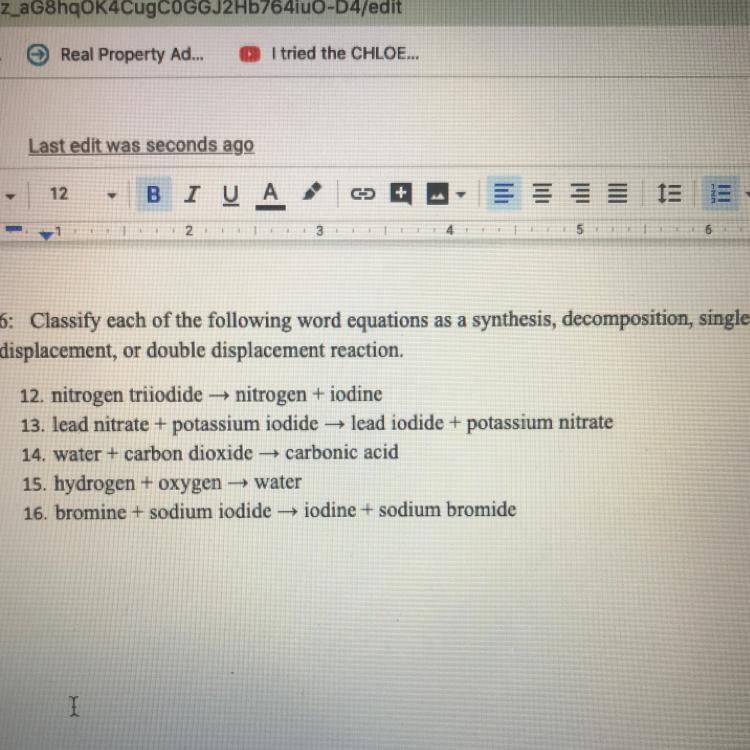
Answers
Answer:
12: This is decomposition because nitrogen triiodide is breaking apart.
13: This is double displacement because the elements in the compounds are "switching".
14: This is synthesis because the water and carbon dioxide are combining.
15: This is also synthesis because the hydrogen and oxygen are combining.
16: This is single displacement because the sodium is "switching" the element it's bonding with.
Give reason that extraction is said to be a reduction process
Answers
Metal extraction is a reduction process because the extracted metal is in a bound state and has a positive valence. The addition of electrons converts cations into atoms or metals.
Metal extraction is always a reductive process. Metals have positive valences in the bound state, and cations are converted to atoms or metals by the addition of electrons. So by definition it is a reductive process.
Example - Mⁿ⁺ + ne⁻ → M
Mining is the process of extracting metal ores buried deep underground. Metal ores occur in varying amounts in the Earth's crust. By extracting metals from ores, minerals in the ground can be used. Ore is very different from the finished metal you see in buildings and bridges. Ore consists of the desired metallic compounds and impurities and a geological material called gangue. The recovery of metals and their separation takes place in several main steps.
Ore Concentration - Here the ore is separated from soil impurities.Separation of metals from concentrated ores – Here the ores are converted to their oxide form and then reduced. The steps involved are either calcination or roasting, followed by heating with a reducing agent.Metal Purification – Here the metal is purified for practical purposes.Learn more about Metal extraction here : https://brainly.com/question/333741
#SPJ9
What are things animals might do to survive?
Answers
Answer:
Animals need food, water, shelter, and space to survive. Herbivores can live only where plant food is available. Carnivores can live only where they can catch their food. Omnivores can live in many places because they eat both plants and animals.
What do the plateau (flat) sections of the graph indicate?A. That a phase change is occurring.B. That heat is being absorbed by the molecules.C. All of theseD. That the temperature is not increasing.

Answers
Answer: the best option to answer the question is letter C, "All of these"
Explanation:
The question requires us to choose the best option about the plateau (flat) sections of heating curve provided.
The graph provided shows the change in temperature in function of time, considering a solid substance (at the beginning of the curve) that is being heated. When enough heat is provided to this solid, it reaches the temperature where the solid can transform into a liquid - its melting point. At this point, the phase change occurs and the temperature does not increase. On the other hand, the substance needs to absorb energy to "promote" the phase change. This transformation happens at the first plateau shown in the graph.
Increasing the temperature, the liquid now is being heated until it reaches the temperature where it can transform from liquid to gas - the boiling point. Similarly to the previous flat section, the phase transformation (liquid -> gas) will occur at a constant temperature and the susbtance needs to absorb enough energy to change its physical state.
Further increasing the heat, the temperature of the substance in gas form is now increasing.
Considering the explanation above, we can say that at the flat sections of the graph, there is a phase transformation ocurring, the temperature does not increase (it is kept constant during the phase transformation) and the substance is absorbing heat to "promote" the change transformation.
Therefore, the best option to answer the question is letter C, "All of these".
What are two types of fibres obtained from the fleece of a sheep? Which one is used to make wool?
Answers
Answer:
Answer: The two types of fibres obtained from the fleece of a sheep are beard hair, which are coarse and fine, and soft under hair, which grow near the skin. The under hair are used to make wool.
Explanation:
mark brainly please!
(I didn't copy the person above me! I just realized we had the same answer.)
can you help me with this problem? just tell me what number is it closer to.

Answers
The atomic mass of magnesium should be closer to 25
How do I determine the atomic mass of magnesium?The following data were obtained from the question:
Mass of 1st isotope = 23.98504 amuAbundance of 1st (1st%) = 78.70%Mass of 2nd isotope = 24.98584 amuAbundance of 2nd (2nd%) = 10.13%Mass of 3rd isotope = 25.98259 amuAbundance of 3rd (3rd%) = 11.17%From the above data, we can see that the 1st isotope (with mass of 23.98504 amu) has an abundance of 78.70% which the greatest compared to the other isotopes.
Thus, the atomic mass of the magnesium will be around 24.
Therefore, we can say that the atomic mass of the magnesium will be closer to 25
Learn more about average atomic mass:
https://brainly.com/question/24185848
#SPJ1
A rectangular container of air measures 1.2 x 10-2 m by 8.34 x 10 1 m by 6.0 x 10-1 m. Air
has a density of 1180 g/m 3. Find the mass of the air in grams.
Answers
The volume of the rectangle has been given by the product of length, breadth, and height. The mass of the air with a density of 1180 g/m³ is 708.57 gms.
What is density?The density of the substance has been known to be given by the mass's ratio to the volume. The formula for density is given as,
Density = Mass ÷ Volume
Given,
Density = 1180 g/m³
The volume of the rectangle = L×B×H
Substituting the values above,
Mass = Density × Volume
M = 1180 × 1.2 × 10⁻² × 8.34 × 10¹ × 6.0 × 10⁻¹
= 708.57 gm
Therefore, the mass of the air is 708.57 gms.
Learn more about density here:
https://brainly.com/question/952755
#SPJ1
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
If H3O+ is an acid according to Brønsted-Lowry, what is its conjugate base?
Select one:
Need ASAP please
a. H4O2+(aq)
b. H+(aq)
c. H2O(aq)
d. OH-(aq)
Answers
Answer:
C. H20(aq)
Explanation:
1 mole of sulfur atoms has how much mass
Answers
Answer:
One atom of sulfur has a mass of 32.07 AMU; one mole of S atoms has a mass of 32.07 g.
Explanation:
Therefore, the answer should be 32.07 g
Which treatment(s) will help remove contaminants from minerals or from the pipes carrying water from a source? you can select more than one (Water Contamination Gizmos) **ONLY ANSWER IF YOU ACTUALLY KNOW ❗️❗️**
answer choices:
Sedimentation
Disinfection
Filtration
Coagulation

Answers
Sedimentation, filtration, and coagulation are the treatments that will help remove contaminants from minerals or from the pipes carrying water from a source.
Sedimentation is a process in which suspended particles settle out of water. It is one of the most basic techniques for removing particles from water. As particles settle, they become trapped in the bottom of a container or settle to the ground in an outdoor setting
Filtration is a method of removing particles from a fluid. It is a physical or chemical separation method that separates solids from fluids (liquids or gases) by adding a medium through which only the fluid can pass.
Coagulation is the process of using chemicals to remove contaminants from water. By creating a chemical reaction, coagulation destabilizes particles and causes them to clump together. This helps to remove the contaminants from the water.
Disinfection is the process of eliminating or destroying pathogens that cause infection. Disinfection eliminates harmful microorganisms by destroying or inactivating them. The disinfectant is a chemical or physical agent that is used to destroy or inactivate harmful microorganisms.
Know more about Filtration here:
https://brainly.com/question/29756050
#SPJ8
Select all that apply
Choose all the statements that correctly describe the process of writing a chemical equation for a physical process.
The representation of the physical state of the substance before the process occurs is written above the arrow.
The representation of the physical state of the substance after the process occurs is found on the product side of the reaction.
The representation of the physical state of the substance before the process occurs is written to the left of the arrow.
If the physical process involves dissolving a substance in water, the water is always shown on the left side of the arrow.
Answers
NiNo3 compound name?
Answers
Answer:
Nickel Nitrate
A wave like the one shown in the diagram below is called a transverse wave. Such a wave is typical of light waves and other types of electromagnetic waves. Every transverse wave has certain properties, including wavelength. One measure of wavelength is the distance from B to D.
Answers
Answer: transmits yellow light
Explanation:
What do we need to know to understand the formation of a chemical bond?
Answers
Answer:
A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds.
Explanation:
You have to put energy into a molecule to break its chemical bonds. The amount needed is called the bond energy. After all, molecules don't spontaneously break
Which organelles is found in plant and animal cells and is involved in making cellular energy?
Answers
Answer:
The plant and animal cells are eukaryotic and contain well developed cellular organelles.
The cell membrane, cytoplasm, chromosomes, and mitochondria are the structures that are present in both the plant and the animal cells.
The cell wall and chloroplast are present only in the plant cell.
Mitochondria
Because it is right next to the nucleau
What is the density of a substance that weighs 340g and has a volume of 40cm3?
Answers
Answer:
8.5 g/cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question we have
\(density = \frac{340}{40} = \frac{17}{2} \\ \)
We have the final answer as
8.5 g/cm³Hope this helps you
The only naturally occurring isotopes of nitrogen are N-14 and N-15.
Based on the atomic mass of the element nitrogen on the Periodic Table, compare the relative abundances of the naturally occurring isotopes of nitrogen
Answers
Answer:
Isotope N–14 = 99%
Isotope N–15 = 1%
Explanation:
Let isotope A be N-14
Let isotope B be N-15
From the question given above, the following data were obtained:
For isotope A (N-14):
Mass of A = 14
Abundance of A = A%
For isotope B (N-15):
Mass of B = 15
Abundance of B = (100 – A%)%
Atomic mass of nitrogen = 14.01 amu
Thus, we can obtain the relative abundances of the naturally occurring isotopes of nitrogen as illustrated below:
Atomic mass = [(Mass of A × A%)/100] + [(Mass of B × B%)/100]
14.01 = [(14 × A%)/100] + [(15 × (100 – A%)/100]
14.01 = 0.14A% + 0.15(100 – A%)
14.01 = 0.14A% + 15 – 0.15A%
Collect like terms
14.01 – 15 = 0.14A% – 0.15A%
– 0.99 = – 0.01A%
Divide both side by – 0.01
A% = – 0.99 / –0.01
A% = 99%
Abundance of B = (100 – A%)%
Abundance of B = (100 – 99)%
Abundance of B = 1%
Thus, the relative abundances of the naturally occurring isotopes of nitrogen are:
Isotope N–14 = 99%
Isotope N–15 = 1%
Diffrentiate the reactivity of metals and Non-metals with air / oxygen.
Answers
Answer:
Both metals and non-metals when burnt in oxygen form their oxides. Oxides of metals are basic in nature and oxides of non-metals are acidic in nature.
50 POINTS!!! Please help! It's urgent! Please explain in detail.
Some household cleaners come in concentrations stronger than necessary for basic cleaning jobs. Jeremy followed the instructions on a cleaning bottle and mixed enough cleaner with 4.0 L of water to form a 1.0 M cleaning solution. After testing his cleaning solution, he decided he should double to concentration for a tough stain. Jeremy added the same amount of cleaner and then added another 4.0 L of water to his bucket. Did Jeremy succeed in making a more concentrated solution? Explain why or why not.
Answers
No. Jeremy did not succeed in making the solution more concentrated.
DilutionInitially, Jeremy mixed an amount (x) of the cleaner with 4. 0 L of water making a concentration of 1.0 M.
x/4 = 1.0 M
Thereafter, he added the same amount of (x) of the cleaner and added another 4.0 L of water:
Total amount of cleaner = x + x
= 2x
Total amount of water = 4 + 4
= 8.0 L
Concentration of the resulting solution = 2x/8
= x/4
x/4 = 1.0 M
Thus the concentration remains the same.
More on dilution can be found here: https://brainly.com/question/13844449?referrer=searchResults
50 points
Which elemental family receives electrons in an ionic bond?
Responses
noble gases
metals
halogens
nonmetals
Answers
Answer: es no metales
Explanation:
A galvanic cell is powered by the following redox reaction: (aq) (aq) (aq)(s) (l) (l) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. Round your answer to decimal places.
Answers
Answer:
The redox reaction in the question is missing. The reaction is :
Cl 2(g) + Mn 2 +(aq) + 2 H-On â 2 Cl-(aq) + MnO2(s) + 4 H + (aq)
2.59V
Explanation:
A redox reaction is also known as oxidation - reduction reaction. In redox reaction, there is a transfer of electrons between the species. In one species there is oxidation and in the other species there is reduction process.
Cathode half reaction equation:
\($ Cl_2(aq) + 2e \rightarrow 2Cl^- (aq)$\)
Anode half reaction equation:
\($ Mn^2 (aq) + 2H_2O (l) \rightarrow MnO_2 (s) +2e +4H\)
E°cathode= 1.36V
E°anode= -1.23V
E°cell= E°cathode - E°anode
E°cell= 1.36 - (-1.23)
E°cell= 2.59V
Match the solution with the correct concentration.

Answers
Answer:
1. is Molar (with capital M)
2. is molal (m)
Explanation:
By definition, 1 Molar solutions have 1 mol of solute in 1 L of solution and 1 molal solutions have 1 mol of solute in 1 Kg of solvent
Please help! Due today!



Answers
Answer:
five(5) of them
sorry if I'm wrong
The enthalpy of vaporization for methanol is 35.2 kJ/mol. Methanol has a vapor pressure of 1 atm at 64.7 oC. Using the Clausius-Clapeyron equation, what is the vapor pressure for methanol at 55.5 oC? Give your answer in atmospheres, to the third decimal point.
Answers
Answer: 55.5 oC is 0.014 atm (3rd decimal point)
Explanation:
The Clausius-Clapeyron equation is given as:
ln(P2/P1) = -(ΔH_vap/R) * (1/T2 - 1/T1)
where:
P1 = vapor pressure at temperature T1
P2 = vapor pressure at temperature T2
ΔH_vap = enthalpy of vaporization
R = gas constant = 8.314 J/(mol*K)
Converting the enthalpy of vaporization to J/mol:
ΔH_vap = 35.2 kJ/mol = 35,200 J/mol
Converting temperatures to Kelvin:
T1 = 64.7 + 273.15 = 337.85 K
T2 = 55.5 + 273.15 = 328.65 K
Substituting the values into the equation and solving for P2:
ln(P2/1 atm) = -(35,200 J/mol / 8.314 J/(mol*K)) * (1/328.65 K - 1/337.85 K)
ln(P2/1 atm) = -4.231
P2/1 atm = e^(-4.231)
P2 = 0.014 atm
Therefore, the vapor pressure for methanol at 55.5 oC is 0.014 atm, to the third decimal point.